Figure 6.
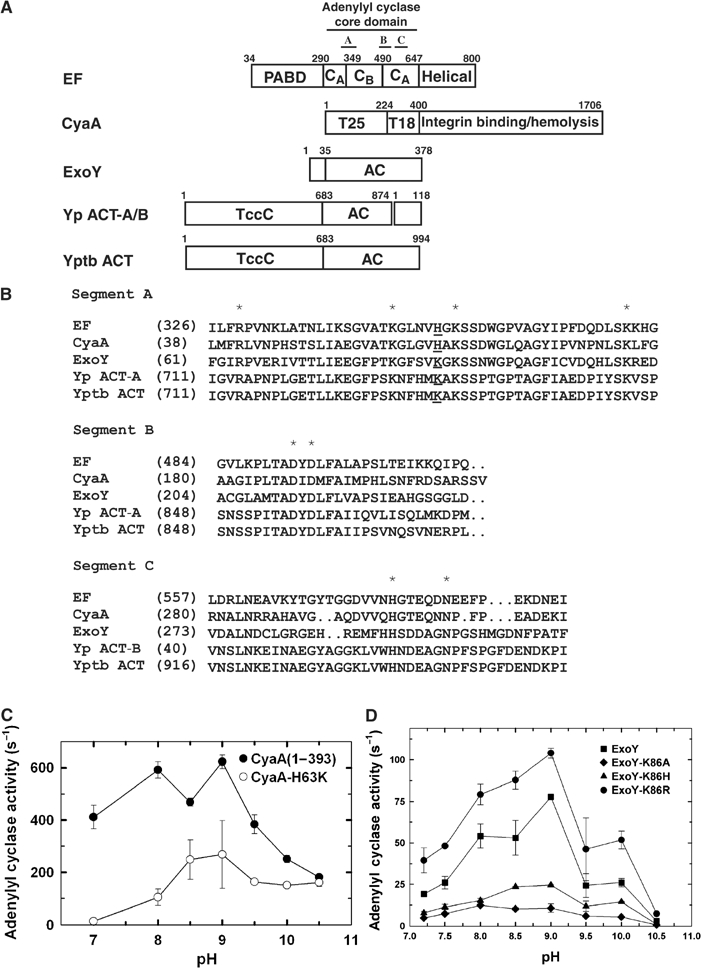
The comparison of class II adenylyl cyclase toxins. (A) Schematic diagram of domain organization of five adenylyl cyclase toxins. The adenylyl cyclase core domains and the conserved segments, A, B, and C are indicated. Yp ACT-A/B and Yptb ACT refer to the adenylyl cyclase toxin from Yersinia pestis and Yersinia pseudotuberculosis, respectively. Accession numbers for EF, CyaA, ExoY, Yp ACT-A, Yp ACT-B, and Yptb ACT are P40126, P15318, AAC78299, NP993432, NP993433, and YP070748, respectively. Yp ACT consists of two polypeptide chains due to the frameshift mutation. Yp ACT-B has sequence homology with bacterial DNA gyrase and oligopeptide ATP transporter. The C-terminal 145 kDa domain of CyaA can bind αMβ2 integrin and has hemolytic activity (El-Azami-El-Idrissi et al, 2003). The N-terminal domains of Yptb and Yp ACT are homologous to the C locus of insecticidal toxin complex c (TccC) (Bowen et al, 1998). (B) Sequence alignment of three conserved segments of class II adenylyl cyclases. The highly conserved residues crucial for catalysis are highlighted by asterisk. EF H351 and the corresponding residues are underlined. The adenylyl cyclase activity of CyaA (C), ExoY (D) and their mutants in response to the pH changes. Adenylyl cyclase activity was measured in the presence of 10 mM ATP, 1.2 μM free CaCl2, 500 μM BAPTA, 10 mM MgCl2, 1 μM CaM (for CyaA) or 10 μg spleen lysate (for ExoY).
