Figure 6.
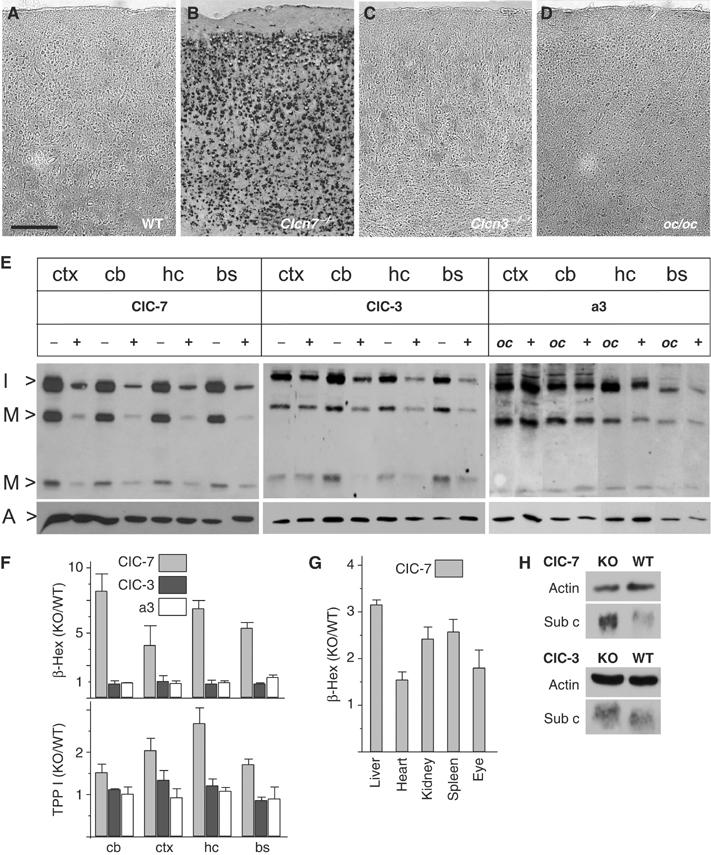
Alteration of lysosomal enzymes in Clcn7−/−, but not in oc/oc or Clcn3−/− mice. (A–D) Lysosomal acid phosphatase activity was visualized in situ as dark staining on cortex sections of p40 WT (A), p40 Clcn7−/− (B), 3 months Clcn3−/− (C) and p29 oc/oc mice (D). Scale bar: 20 μm. (E) Immunoblot analysis of cathepsin D. Extracts of different brain regions (ctx, cortex; cb, cerebellum; hc, hippocampus; bs, brainstem) of p30 Clcn7−/−, Clcn3−/− and oc/oc mice were compared to WT controls. Bands represent the intermediate (I>, 42 kDa) and the mature forms (M>, 32 and 12 kDa) of cathepsin D. Staining for actin (A>) served as a loading control. (F) Enzymatic activity of β-hexosaminidase (β-Hex) and TPP I in brain extracts from Clcn7−/− (gray bars), Clcn3−/− (black bars) and oc/oc mice (white bars) at p30 (shown is the ratio of mutant over WT; error bars, s.e.m.; n=4). (G) Increase of β-hexosaminidase activity in the eye and peripheral organs from Clcn7−/− mice. (H) Western blot analysis of subunit c of the mitochondrial ATP synthase of total brain homogenates from Clcn7−/−, Clcn3−/− and WT mice. Actin served as loading control.
