Figure 3.
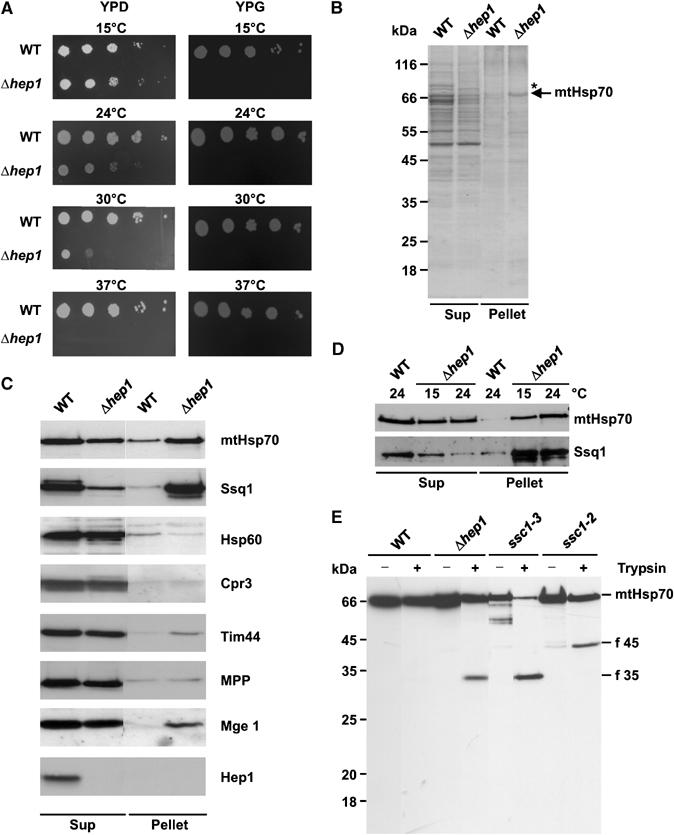
Deletion of HEP1 leads to aggregation of mitochondrial Hsp70 proteins. (A) Drop dilution test of WT and a HEP1 deletion strain (Δhep1). Dilutions, 10-fold, were plated on YPD and YPG plates and incubated at the indicated temperatures. (B, C) Aggregation of mtHsp70 in cells lacking Hep1. Mitochondria isolated from WT and Δhep1 cells were lysed with 1% digitonin and aggregated material was pelleted by centrifugation (Pellet). The SN fraction (Sup) was precipitated with TCA. Pellets and Sups were analysed by SDS–PAGE and Coomassie staining (B) or immunodecoration with antibodies against mtHsp70, Ssq1 and the other indicated proteins (C). (D) Aggregation of Hsp70 proteins depends on the growth temperature of cells. WT and Δhep1 cells were grown at 15°C and 24°C. Then, mitochondria were isolated and the aggregation state of mtHsp70 and Ssq1 was tested as above. Equal amounts of protein from the Pellet and Sup fraction were analysed. (E) Increased protease sensitivity of mtHsp70 in the absence of Hep1. Mitochondria were isolated from WT, from Δhep1 cells and from temperature-sensitive mutants, ssc1–3 and ssc1–2, grown at permissive temperature. Mitochondria were lysed in the presence of Triton X-100 and treated with trypsin for 5 min on ice. Samples were analysed by SDS–PAGE and immunodecoration with antibodies against full-length mtHsp70. The obtained fragments are indicated: f 45, N-terminal fragment; f 35, C-terminal fragment.
