Figure 4.
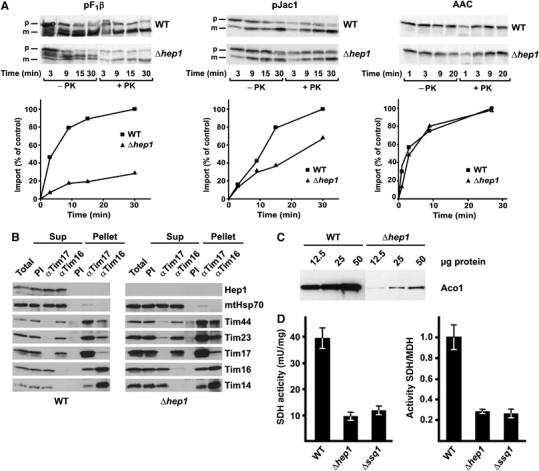
Cells lacking Hep1 are defective in import of mitochondrial preproteins and in the biogenesis of FeS proteins. (A) Mitochondria isolated from Δhep1 cells and from WT were incubated with radiolabelled preproteins. The substrates pF1β (subunit β of the F1Fo-ATPase) and pJac1 of the TIM23 complex and the substrate of the TIM22 complex, ATP/ADP carrier (AAC), were used as preproteins. Mitochondria were treated with proteinase K, reisolated and analysed by SDS–PAGE and autoradiography. The mature forms of the proteins were quantified. Import into WT mitochondria at the longest time point was set to 100%. p, precursor form; m, mature form. (B) Hep1 is not part of the TIM23 complex. Mitochondria were isolated from WT cells (left panel) and cells lacking Hep1 (right panel). After solubilization with digitonin, the lysate was subjected to immunoprecipitation with antibodies against Tim17, Tim16 and with preimmune serum (PI). Samples were analysed by SDS–PAGE and immunodecoration with antibodies against the indicated proteins of the TIM23 complex and Hep1. ‘Total' and ‘SNs' represent 20% of material present in ‘Pellets'. (C) Mitochondrial proteins were analysed by SDS–PAGE and immunodecoration with antibodies against aconitase. (D) The activities of SDH and malate dehydrogenase (MDH) were measured in mitochondria from WT, Δhep1 and Δssq1 cells. The SDH activities are given per mg of mitochondrial protein (left panel) and relative to the activity of malate dehydrogenase (right panel). The ratio obtained for WT mitochondria was set as 1. Error bars show the standard deviation of three independent experiments.
