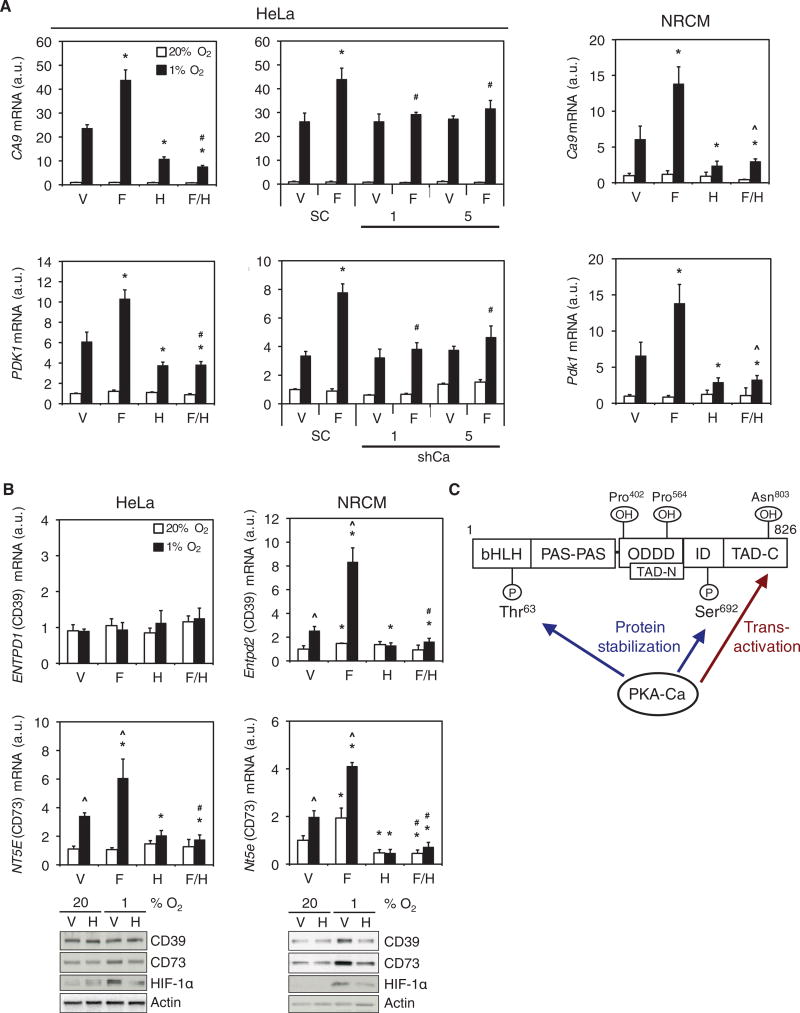
Fig. 6. PKA stimulates HIF-1 target gene expression.
(A) CA9 and PDK1 mRNA expression was determined by RT-qPCR in: parental HeLa cells; scrambled shRNA control (SC), shCa-1 or shCa-5 HeLa subclones; and NRCMs exposed to vehicle (V), forskolin (F), H89 (H), or forskolin+H89 (F/H) at 20% or 1% O2 (mean ± SD, n = 6 biological replicates from 2 independent experiments). *p < 0.05 compared to V; #p < 0.05 compared to SC; ^ p < 0.05 compared to H. (B) CD39 and CD73 mRNA expression and the abundance of the corresponding proteins in HeLa cells or NRCMs exposed to V, F, H, or F/H at 20% or 1% O2 were determined by RT-qPCR (mean ± SD, n = 6 biological replicates from 2 independent experiments) and immunoblot assays (n = 3 independent experiments). ^p < 0.05 compared to 20% O2; *p < 0.05 compared to V; #p < 0.05 compared to H. Data in A and B are graphed as fold change from respective controls at 20% O2. The Mann-Whitney U test was used to assess statistical significance for all data in A and B. (C) PKA regulates HIF-1α protein stability and carboxy-terminal transactivation domain (TAD-C) function. Phosphorylation of Thr63 and Ser692 by PKA inhibits proteasomal degradation of HIF-1α. PKA also stimulates p300 binding to overcome the inhibitory effect of FIH-1-mediated Asn803 hydroxylation, thereby increasing transactivation by HIF-1α.