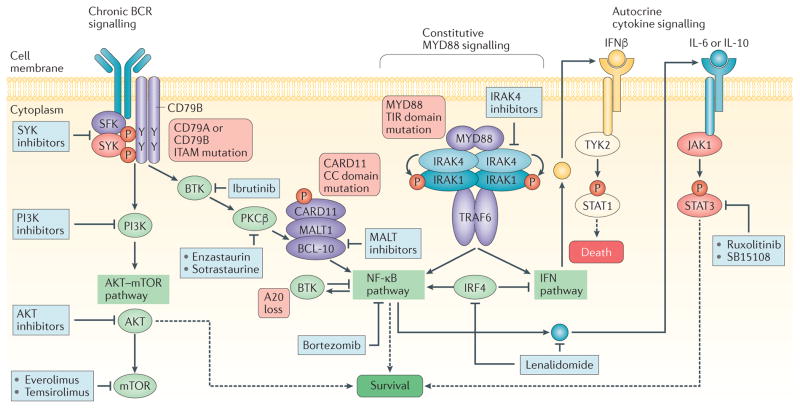
Figure 3. Diverse genetic alterations in upstream pathways contribute to aberrant NF-κB activity in DLBCL.
Systematic analysis of genes in pathways upstream of the nuclear factor-κB (NF-κB) complex revealed a large repertoire of diffuse large B cell lymphoma (DLBCL)-specific genetic alterations in B cell receptor (BCR) and myeloid differentiation primary response 88 (MYD88) pathways. The presence of these mutations leads to aberrant activation of the canonical p50-RELA heterodimer and associated tumour dependency. These mutations, which are more frequent in the activated B cell (ABC) subtype of DLBCL, have provided the rationale for the clinical development of several BCR pathway inhibitors, such as ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor. CARD11, caspase recruitment domain family member 11; IFN, interferon; IL, interleukin; IRAK, IL-1 receptor-associated kinase; IRF4, interferon regulatory factor 4; ITAM, immune receptor tyrosine-based activation motif; JAK1, Janus kinase 1; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; PKC, protein kinase C; STAT, signal transducer and activator of transcription; TIR, Toll-interleukin receptor; TRAF6, TNF receptor associated factor 6. Adapted with permission from REF. 134, Nature Publishing Group.