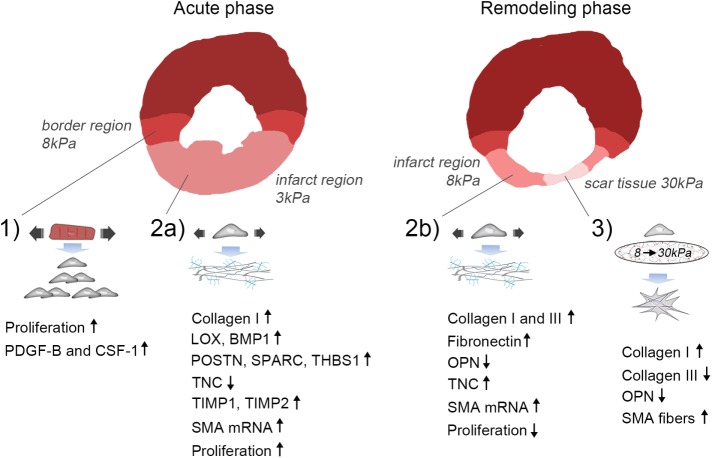
FIGURE 5:
Proposed model of mechanical regulation of CFBs following MI. 1) Paracrine signaling involving PDGF-B and CSF-1 from stretched CMs in the border region leads to proliferation of CFBs. 2a) Stretch of the infarct region during the acute phase after MI (ECM stiffness ∼3 kPa) promotes collagen I, LOX, BMP-1, POSTN, SPARC, THBS1, TIMP1 and 2, while inhibiting TNC expression by CFBs. Proliferation is increased during this phase. 2b) Stretch of the infarct region during the remodeling phase (ECM stiffness ∼8 kPa) also up-regulates collagen I in addition to collagen III, FN, and TNC, while it down-regulates the matricellular protein OPN. Proliferation is decreased during this phase. Stretch causes up-regulation of SMA mRNA during both the acute and remodeling phases; however, incorporation into stress fibers requires an accompanying increase in matrix stiffness. 3) At late stages of infarct healing, ECM stiffening to pathological stiffnesses (∼30 kPa) dominates the mechanical environment. This promotes a contractile CFB phenotype due to SMA fiber formation that has elevated, albeit dampened, collagen I production compared with that of stretched CFBs. Collagen III and OPN expression is reduced, supporting scar maturation and healing. However, continuous stretch of CFBs at this stage will cause persistent ECM production and thus development of pathological fibrosis.