A nanofilm of cross-linked collagen-I is equivalent to a relatively stiff matrix, which stiffens the nucleus, correlating broadly with lamin-A (including mutant progerin), retinoic acid transcription factor level and activity, and osteoinduction. In vitro results are supported by studies of ectopic bone formation in vivo.
Abstract
Synergistic cues from extracellular matrix and soluble factors are often obscure in differentiation. Here the rigidity of cross-linked collagen synergizes with retinoids in the osteogenesis of human marrow mesenchymal stem cells (MSCs). Collagen nanofilms serve as a model matrix that MSCs can easily deform unless the film is enzymatically cross-linked, which promotes the spreading of cells and the stiffening of nuclei as both actomyosin assembly and nucleoskeletal lamin-A increase. Expression of lamin-A is known to be controlled by retinoic acid receptor (RAR) transcription factors, but soft matrix prevents any response to any retinoids. Rigid matrix is needed to induce rapid nuclear accumulation of the RARG isoform and for RARG-specific antagonist to increase or maintain expression of lamin-A as well as for RARG-agonist to repress expression. A progerin allele of lamin-A is regulated in the same manner in iPSC-derived MSCs. Rigid matrices are further required for eventual expression of osteogenic markers, and RARG-antagonist strongly drives lamin-A–dependent osteogenesis on rigid substrates, with pretreated xenografts calcifying in vivo to a similar extent as native bone. Proteomics-detected targets of mechanosensitive lamin-A and retinoids underscore the convergent synergy of insoluble and soluble cues in differentiation.
INTRODUCTION
Stem cells differentiate in response to microenvironmental cues that derive from surrounding matrix, cell contacts, and soluble factors (Fuchs et al., 2004; Engler et al., 2006; Nelson and Bissell, 2006), but synergy and convergence between cues is understudied (Figure 1A). Mesenchymal stem cells (MSCs) are classically isolated by adhesion to rigid plastic (Pittenger et al., 1999) but are clearly affected in differentiation by the stiffness of a synthetic gel with matrix ligand (Engler et al., 2006), as well as by many soluble factors, such as retinoic acid (RA; Swift et al., 2013b) which widely regulates RA receptor (RAR) transcription factors in differentiation (Williams et al., 2009). MSCs in bone marrow contribute to osteogenesis (Park et al., 2012), but they also reside in many tissues within perivascular niches, where they contribute to fibrosis, and some differentiated lineages (Kramann et al., 2015), as well as neoplasms (Medyouf et al., 2014). Many recent studies of differentiation and disease with various stem cells and gels (e.g., Musah et al., 2014) have left unaddressed whether one key physiological modification that should stiffen matrix, namely enzymatic cross-linking, can affect the differentiation effects of equally physiological soluble factors such as RA. Stiffening of bulk matrix by enzymatic cross-linking affects cancer cells in vitro and in vivo (Cox et al., 2013), but differentiation studies at the single-cell scale with nanocontrol of cross-linking could provide deeper insight into synergy of matrix elasticity with a potent soluble factor such as RA.
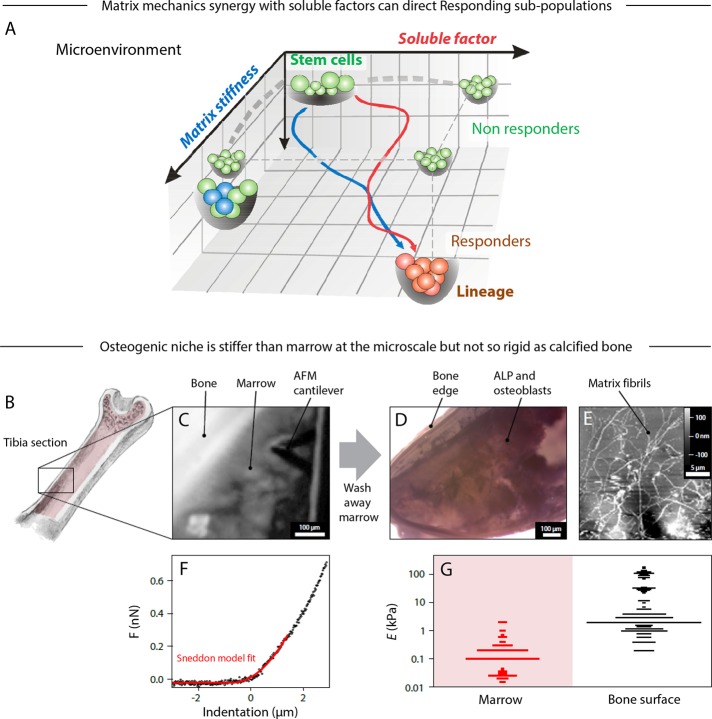
FIGURE 1:
The bone microenvironment defines mechanical and molecular properties that influence the trajectory of stem cell differentiation. (A) The fate of a stem cell on a Waddington-like landscape is potentially influenced by matrix properties in synergy with soluble factors. One example is osteogenesis of bone marrow MSC. However, differentiation might also include subpopulations that are not responsive to either stimuli or their combination. (B) Mouse trabecular bone was cut and opened to expose the tissue inside for probing by AFM. (C) Bright-field image showing bone marrow probed in situ by AFM. The bone marrow was subsequently washed away and the remaining tissue and osteoid reexamined. (D) Bone tissue stained for ALP activity. (E) Contact-mode AFM image of the exposed bone, showing extracellular matrix fibers as part of the osteoid. (F) A typical force–indentation curve of bone marrow (prewash), fitted with a modified Hertz model for a conical probe (red). (G) Comparison of the distributions of Young's modulus, E, measured by AFM at different locations in the prewashed marrow, which is soft, and exposed osteoid, which is typically stiffer. All experiments, n ≥ 3 (mean + SEM).
Collagen-I is not only the most abundant protein in animals and a well-known target of enzymatic cross-linking, but it is also intrinsically proosteogenic (Yener et al., 2008). Cells attach to collagenous matrix and use actomyosin forces to pull on it (Discher et al., 2005), with stiff matrices driving cytoskeleton assembly and cell spreading within hours (Engler et al., 2006). Nuclei flatten and also spread with the cell, and the level of the “keratin-like” nuclear protein lamin-A increases, consistent with lamin-A (but not lamin-B) being high in collagen-rich tissues such as bone but low in soft tissues with low collagen such as marrow and brain (Swift et al., 2013b). Lamin-A mutations affect many stiff tissues, as illustrated by defects in muscle, bone, and skin in the accelerated aging syndrome progeria, whereas soft tissues remain unaffected (Bridger et al., 2007). Such observations suggest matrix-linked regulatory roles for lamins, and recent surprising results for lamin levels in MSCs on gels further show that retinoid regulation occurs only with cells on stiff substrates as opposed to soft gels (Swift et al., 2013b). Promoter-reporter approaches have already shown that the promoter region of the LMNA gene binds RAR transcription factors (Okumura et al., 2004), so that changes in lamin-A message can be caused by at least some retinoids in culture. However, it is unclear which of the three known RAR isoforms (RARA, RARB, and/or RARG) and which (if any) isoform-specific soluble factors are relevant to regulation in MSCs, especially when cultured on physiological matrices with enzymatic cross-linking.
Here stiffness measurements of the tibia's osteogenic niche are followed by meta-analyses of transcriptomes from tissues that span a wide range of stiffness and that suggest general associations of collagen-I with matrix cross-linking, myosin contractile forces, lamin-A, RARG, and osteogenic induction. These tissue-level associations are all observed in culture with naive MSCs on collagen-I nanofilms that are cross-linked rather than pristine, and the results map into gels of suitable effective stiffness. RARG's nuclear localization primarily in cells on stiff substrates helps to explain the rigidity-dependent regulation of lamin-A expression by soluble RARG agonists/antagonists, and the findings are extended to lamin-A's “progerin” splice-form in induced pluripotent stem cell (iPSC)–derived MSCs from a progeria patient (Olive et al., 2010). Matrix rigidity synergizes with RARG agonist suppression of normal and progerin lamin-A, and rigidity also synergizes with RARG antagonist to increase lamin-A and enhance osteogenesis in vitro and in vivo. The approach is likely relevant to fibrosis of the perivascular niche (Kramann et al., 2015), in which a thin layer of matrix is enriched in cross-linked collagen-1 to drive—in the absence of osteogenic soluble factors—a fibrogenic phenotype with high actomyosin contractility, lamin-A, and RARG (Dingal et al., 2015).
RESULTS
Cell-scale stiffness of the osteogenic niche
In bone formation, MSCs egress from a niche that is likely perivascular and migrate to a precalcified surface in differentiation to osteoblasts (Park et al., 2012). In vitro studies show that matrix stiffness directs MSC migration (Raab et al., 2012) as well as differentiation (Engler et al., 2006), and yet no measurements exist for the in situ mechanical properties of osteoid matrix at the microscale to which a cell adheres and probes. For cartilage, atomic force microscopy (AFM) has been used (Stolz et al., 2009) to measure an interstitial elastic modulus E at a scale that approximates that of the matrix surrounding chondrocytes (Guilak et al., 2005) but also proves to be 10-fold or more softer than the macroscopic rigidity of cartilage.
To study the osteogenic niche in bone, we sliced mouse tibia along its length (Figure 1B) and applied AFM nanoindentation (Figure 1C) to the exposed marrow and then to the inner surface of bone after washing away marrow. Samples were fixed and stained for alkaline phosphatase (ALP) activity, which confirmed the presence of osteoblastic cells (Figure 1D), and high-resolution AFM imaging revealed fibrillar matrix (Figure 1E). Force–indentation data (Figure 1F) revealed E for marrow to be ∼0.1 kPa versus a much stiffer bone surface with peaks at 2, 30, and 100 kPa (Figure 1G). The softest peak is close to E for isolated cells of mesenchymal origin (Titushkin and Cho, 2007; Yourek et al., 2007; Darling et al., 2008; Yim et al., 2010). The 30- and 100-kPa peaks could reflect the elastic response and the spatial heterogeneity of the mineralized matrix due to the presence of interfibrillar and extrafibrillar bioapatite (Alexander et al., 2012). Stiffer substances were also detected and likely correspond to calcified bone (on the order of gigapasacals) but cannot be resolved with soft cantilevers. The 30-kPa peak is consistent with E of the osteoid matrix secreted by cultured osteoblasts (Engler et al., 2006). Osteogenesis is thus associated with stiffer tissue than the marrow space that is filled with hematopoietic cells. To address whether such stiff matrix has osteogenic specificity or not, we made a compositional comparison to other collagen-rich tissues.
Proteomics and transcriptomic meta-analysis identify possible factors in MSC osteogenesis
Mass spectrometry (MS) proteomics of bone compared with two other stiff tissues, muscle and skin (Figure 2A, i–iii), showed that all had an abundance of collagen-I (Col1a1 and Col1a2). Collagen-1 is not only the most abundant protein in our bodies (Neuman and Logan, 1950) and a key determinant of tissue mechanics (Swift et al., 2013b), but it is also the main scaffold protein of osteoid that acts together with soluble factors in a niche for MSC osteogenesis and bone mineralization (Katz and Li, 1973; Stein et al., 1990). To provide evidence for such a process, we cultured human MSCs with standard osteogenic soluble factors, engrafted them into mouse flanks, and excised them after 4 wk for profiling by MS. Human-specific peptides were ∼10% of all peptides (Supplemental Figure S1A), and overall profiles (Swift et al., 2013a,b) compare well to MSC cultures (Figure 2A, iv and v), with a matrix complexity similar to mouse bone rather than mouse skin at the graft site. Of interest, periostin is known to activate the cross-linking enzyme lysyl oxidase (Lox), which stiffens collagen (Maruhashi et al., 2010).
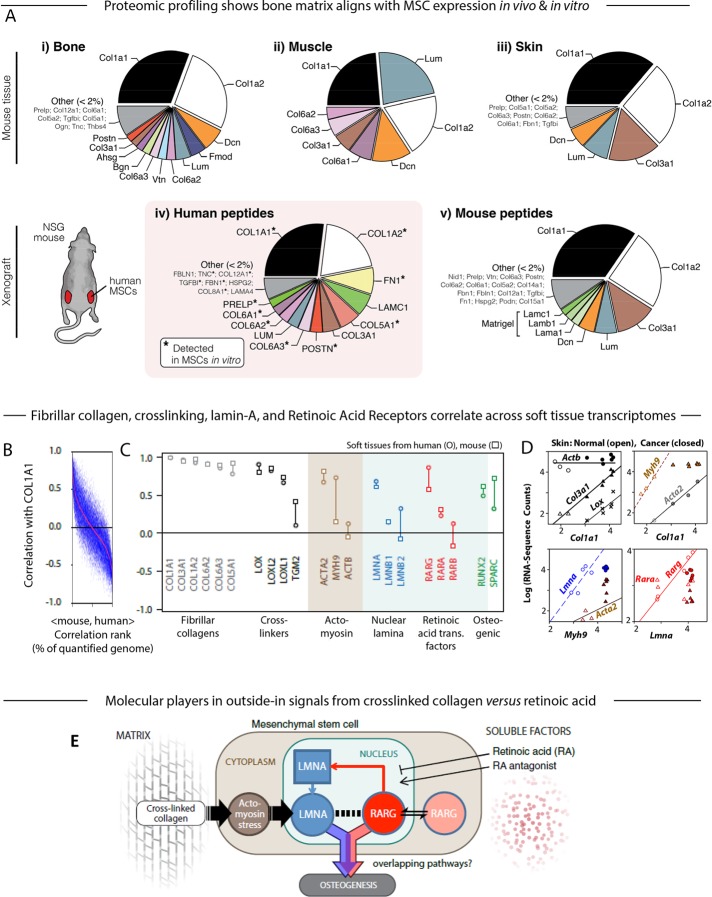
FIGURE 2:
Proteomic and transcriptomic profiles of mouse tissue and human xenografts suggest the key components in a model of the microenvironment-to-osteogenesis signaling cascade. (A) Whole mouse tissues (i, bone; ii, muscle; iii, skin) were profiled by MS proteomics and the ratios of mean ion currents used to estimate the fractional compositions of the respective extracellular matrices. In all cases, the matrix was dominated by collagen-I. Human MSCs were also engrafted into mouse flank, excised after 4 wk, and examined by MS. Subsequent analysis allowed separate identification and quantification of mouse and human peptides (Supplemental Figure S1A; Swift et al., 2013a,b). The human peptides in the xenograft (iv) showed some similarity to the endogenous bone profile, and most of the detectable matrix proteins were also present in preengraftment MSCs (asterisk). Mouse peptides in the xenograft (v) suggested comparison to skin, perhaps unsurprising, given the location of the graft, and also showed likely remnants of the Matrigel component of the initial injection. (B) Pearson correlation between primary matrix component COL1A1 mRNA and other genes quantified in soft tissues of mouse and human (genes with common annotation, n ≈ 15,000), sorted by the mean Pearson coefficient in mouse and human (red line). (C) Pearson correlation between COL1A1 and transcripts for fibrillar collagens, cross-linking enzymes, actomyosin cytoskeleton proteins, nuclear lamina proteins, RAR, and osteogenic transcription factors. Many of these key components were in the top few percent of correlations with collagen-I, as seen by comparison to Figure 2B. (D) RNA-sequencing data from mouse skin of normal or induced squamous cell carcinomas (SCCs; Friedrichs et al., 2007) revealing power-law relations between many of the factors analyzed in C. (E) Gene circuit model of how extracellular factors, mechanical (matrix composition and cross-linking) and molecular (soluble factors such as RA), can influence MSC osteogenesis. Matrix stiffness induces stress in the actomyosin network, which feeds into levels of nucleoskeletal protein LMNA (Buxboim et al., 2014); LMNA regulates its own transcript RARG, which in turn can be modulated by soluble agonists and antagonists to the RA pathway (Swift et al., 2013b). Both LMNA, through regulation of the SRF pathway (Ho et al., 2013; Swift et al., 2013b; Buxboim et al., 2014; Talwar et al., 2014), and the RA pathway conceivably contribute to an osteogenic endpoint, but the extent of overlap between these pathways is not known (Figure 1A). All experiments, n ≥ 3 (mean + SEM).
MS profiling of tissues shows that stiffer tissues have more fibrillar collagen (with bone > muscle > fat > brain), and so for a diverse set of tissues, we conducted a meta-analysis of transcriptomes to ask what transcripts generically associate with collagen-I (COL1A1) in human and mouse (Figure 2B). A wide range of soft through stiff tissues was analyzed because MSCs are found in most tissues beyond marrow (Kramann et al., 2015), and MSCs can be induced toward many tissue lineages. Bone transcriptomes were excluded so that the generic nature of any correlations might be assessed in an unbiased way with MSC cultures and osteoinduction. COL1A1 mRNA scaled with protein across many tissues (Supplemental Figure S1B), and the top few percent of COL1A1-correlated transcripts suggests generic relationships with 1) collagen cross-linking, 2) a tensed actomyosin cytoskeleton, 3) a stiff nucleus with high lamin-A, and 4) osteogenesis (Figure 2C). Fibrillar collagens generally correlate with COL1A1, as do enzymes that cross-link collagen, particularly LOX, but also tissue transglutaminase (TGM2) to a more limited extent. Smooth muscle actin (ACTA2) is a well-known marker of cytoskeleton tension (Wipff et al., 2007), as is nonmuscle myosin-IIA (MYH9), and both correlate with COL1A1, as does nucleoskeletal lamin-A (LMNA), which we showed confers nuclear stiffness (Swift et al., 2013b). No correlations are seen with ubiquitous β-actin (ACTB) or B-type lamins (LMNB1, LMNB2), with the latter consistent with near-constant protein expression across tissues (Swift et al., 2013b). Lamin-A transcription is regulated by RAR transcription factors (Swift et al., 2013b), but COL1A1 correlates only with RARG, which is the one isoform known to contribute to osteogenesis (Williams et al., 2009). Surprisingly, even though bone is not included in this meta-analysis, COL1A1 shows moderate correlations with the early osteogenic transcription factor RUNX2 and with the late osteogenic marker of bone matrix, SPARC (p ≈ 0.5).
Skin transcriptomes from mice were analyzed in order to challenge the foregoing molecular associations and also assess their possible relevance to subcutaneous xenografts (Figure 2A). RNA-sequencing data recently produced from both healthy tissue and chemically induced squamous cell carcinoma (Nassar et al., 2015) show a near linear relation versus Col1a1 for Col3a1, Lox, and Acta2, whereas Actb is constant across both healthy and cancerous skin (Figure 2D). Myh9 also increases with Col1a1 in healthy tissue but remains constant in cancer. For normal tissue but not cancer, Lmna increases with Myh9, and Rarg (but not Rara) increases with Lmna. Therefore, together with our recent findings that stiff matrix promotes actomyosin contractile stress, which stabilizes lamin-A expression in MSCs, the foregoing meta-analyses suggest a hypothesis to test in vitro: if stiff, cross-linked collagen ultimately couples to RARG levels and nuclear localization, then both rigid matrix and RARG-specific soluble factors will jointly modulate lamin-A expression and osteogenesis (Figure 2E).
Enzymatic cross-linking stiffens collagen-I nanofilms to drive symmetric spreading of MSCs
As a minimal culture substrate to use for testing our working hypothesis, we assembled nanofilms of collagen-I on untreated mica substrates (Cisneros et al., 2007) and either left the films pristine or enzymatically cross-linked them (Figure 3A). We used TGM2 for cross-linking because it is broadly expressed (Figure 2C) and required for fibronectin-collagen networks in early matrix genesis (Al-Jallad et al., 2006). TGM2 is also found in bone and in osteoblastic cells associated with matrix mineralization (Heath et al., 2001; Kaartinen et al., 2002). Collagen-I fibrils are only 300 nm long, but self-assembly of these semiflexible rods produces highly ordered fibrillar films that, regardless of cross-linking, exhibit in physiological buffers the expected D spacing of 67 nm (Meek et al., 1979), as well as fibril height of 2.2 or 1.7 nm (Figure 3B, i and iii, and Supplemental Figure S2, A and B), consistent with staggered tropocollagen.
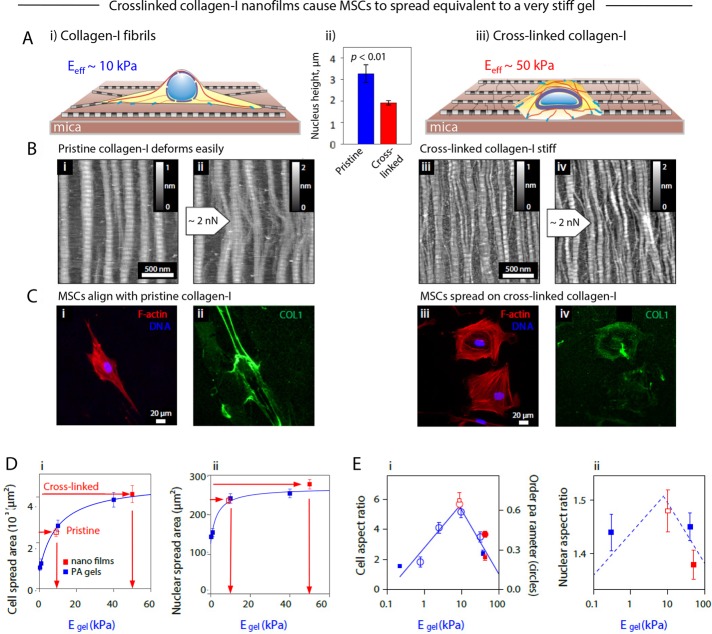
FIGURE 3:
Analysis of cell morphology and protein organization in MSCs cultured on pristine and cross-linked collagen-I films shows the equivalence of cross-linking to increased matrix stiffness. (A) Thin collagen films allowed an in vitro study of the effects on MSC morphology of culture on (i) pristine and (iii) cross-linked collagen-I films; (ii) nuclear height on the two films. (B) AFM amplitude-mode topographical images of molecular films of highly ordered collagen-1 fibrils, showing D-periodic structure, self-assembled on hard substrates in the absence (i, ii) and presence (iii, iv) of cross-linking. Collagen fibrils were deformed by using the AFM stylus in lithography mode to apply a low dragging force across the film (ii, iv), thus showing the flexibility of the fibrils and the deconstruction of the ribbons to monomers in the absence of cross-linking (ii). (C) Immunofluorescence images of MSCs cultured for 24 h on (i) pristine and (iii) cross-linked collagen-I films with staining against F-actin (red) and DNA (Hoechst, blue). (ii, iv) Staining against collagen showed that cross-linked films are not as severely deformed as the native ones. The random orientation of the dense regions of collagen protein suggests that the films are mechanically anisotropic, despite the topographically isotropic orientation of the fibrils evident from AFM images. (D) Comparison of matrix E–dependent cell morphologies of MSCs cultured on thick, isotropic polyacrylamide (PA) gels or thin collagen-I films. Plots of (i) cellular and (ii) nuclear spread area were fitted to hyperbolic functions, with free parameters obtained from cells on gels of controlled stiffness (blue) and cells on thin films (red squares), with the unknown effective stiffness felt by the cells on thin films superimposed to obtain the effective stiffness (10 kPa for pristine and 50 kPa for cross-linked collagen-I films). (E) Cellular aspect ratio (i, left axis), order parameter (i, right axis), and nuclear aspect ratio (ii) as functions of the gel matrix elasticity also showed consistency between morphology of pristine and cross-linked films with 10- and 40-kPa PA gels, respectively. All experiments, n ≥ 3 (mean + SEM).
Nanofilm mechanics were altered by collagen cross-linking. Pristine films are anisotropic, with higher tensile strength in the long axes than in the perpendicular direction (Friedrichs et al., 2007), and fibrils are attached to the atomically flat and rigid mica substrate only by weak electrostatic interactions, so that fibrils are readily displaced and deformed. Lateral forces on fibrils can be exerted moving an AFM tip across the surface while applying a normal force of the order of nanonewtons, which cells can easily exert via their adhesions (Discher et al., 2005; Geiger et al., 2009); subsequent imaging by AFM in tapping mode reveals unraveling of fibrils in pristine films, whereas cross-linked fibrils remain intact, although slightly bent (Figure 3B, ii and iv). Higher normal forces of 20 nN (Supplemental Figure S2, A and B) produced local deformations of nanofilms, which suggested that cross-linking increased film stiffness by at least twofold, consistent with earlier measurements of dialdehyde–cross-linked films (Friedrichs et al., 2007). Slopes of lateral force signal versus distance were similarly higher for cross-linked than for pristine films (Supplemental Figure S2, C and D). As a further check on the effects of TGM2 addition, some MSC xenografts (Figure 2A) that were removed from mice for profiling were treated or not with TGM2 and deformed by micropipette aspiration (Majkut et al., 2013). Cross-linked grafts under such stress relaxed at a slower rate than untreated grafts (Supplemental Figure S1C), which is consistent with a more solid-like response due to cross-linking.
Human bone marrow MSCs that were seeded on the nanofilms showed morphology differences within hours. On pristine nanofilms, immunofluorescence imaging showed predominantly spindle-shaped MSCs (Figure 3Ci and Supplemental Figure S2, C and D), and AFM imaging at submicrometer scales showed cell protrusions and stress fibers aligned with collagen (Supplemental Figure S2E). Immunofluorescent collagen showed that cells displace the flexible network and bundle fibrils into thick fiber bundles (Figure 3Cii) that are expected to withstand large tensions as cells adhere and pull (Friedrichs et al., 2007). On cross-linked nanofilms, in contrast, MSCs exhibited a distinctly well spread and rounded osteo-like morphology (Figure 3Ciii), and collagen was not detectably displaced by cells (Figure 3Civ). Pulling forces exerted by a cell on its matrix have an (equal and) opposite action within the cell in promoting actomyosin network formation and orientation of stress fibers (Zemel et al., 2010). Indeed, cells grown on pristine collagen films show tight alignment of stress fibers that is absent from cells on cross-linked nanofilms (Supplemental Figure S2, C and D).
Cell morphologies map responses on nanofilms into equivalent soft or stiff hydrogels
Synthetic polymer systems of controlled stiffness E are widely reported to drive spreading of diverse cell types (Pelham and Wang, 1997; Engler et al., 2006; Geiger et al., 2009), and such gels provide standards to compare to almost any other new substrate, including a nanofilm. MSCs not only spread more on stiff gels than on soft gels, but they also elongate on an intermediate Egel even though gels are isotropic (Engler et al., 2006; Rehfeldt et al., 2012). The increased cell spread area versus Egel fits a hyperbolic function independent of substrate material (Rehfeldt et al., 2012), and so spread areas of the same MSCs on nanofilms here can be used to infer an equivalent Egel felt by cells (Figure 3Di). MSCs on pristine films thus spread on 10-kPa gels to the same extent, and MSCs on cross-linked films spread the same as on 50-kPa gels.
With fixed cells on stiff substrates, a large value for nuclear spread area (as a projected area) reflects nuclear flattening (Lovett et al., 2013), and for live cells on the nanofilms, our AFM measurements of nuclear height confirm such flattening (Supplemental Figure S2F). Consistent with a simple flattening of the nuclear lamina in spreading on stiff substrates, total lamin-B is independent of matrix stiffness (Supplemental Figure S2Gi). In contrast, lamin-A increases in the same dual-stained MSCs (Supplemental Figure S2Gii). The increased stoichiometry for lamin-A:lamin-B versus microenvironment stiffness is consistent with in vitro and in vivo results for cancer cell lines (Swift et al., 2013b) and with mRNA trends for normal tissues (Figure 2C). Nuclear projected areas are also consistent with the effective elasticities (Figure 3Dii), as are cell and nuclear aspect ratios and stress fiber alignment (Figure 3E, i and ii). Thus, whereas AFM nanoscale measurements of the films suggest a greater than twofold increase in stiffness after cross-linking, cell and nuclear morphology analyses suggest an approximately fivefold increase in the stiffness that cells sense. Of importance, because osteogenesis of MSCs on gels with Egel > 20–40 kPa greatly exceeds that of MSCs on 10-kPa gels (Engler et al., 2006; Dingal et al., 2015), osteoinduction as favored by high lamin-A (Swift et al., 2013b) was next hypothesized to be highest for the cross-linked nanofilms.
Cross-linked nanofilms promote myosin-II, lamin-A, RARG, and osteogenesis
Cells on soft gels are reportedly softer than cells on stiff gels (Solon et al., 2007; Liu et al., 2014; Pagliara et al., 2014; Staunton et al., 2016), but any cell indentation device such as an AFM in reality measures the effective elasticity of the cell and gel below it, analogous to two springs in series (Staunton et al., 2016). The ∼2-nm films here are ∼1000-fold thinner than cells and are on rigid substrates, and so moderate indentations reveal a cell's elasticity without the need for complex decoupling of the mechanical contribution of the substrate (Figure 4A). Force–volume maps of MSCs adhering to either pristine or cross-linked nanofilms thus reveal apparent cell elasticities in the kilopascal range, with the nucleus seeming to be stiffer than perinuclear regions (Figure 4B). By probing many MSCs in the nuclear region (∼1 μm deep), this apparent nuclear stiffness of cells on cross-linked nanofilms proves approximately twofold higher than for cells on pristine collagen films (Figure 4C).
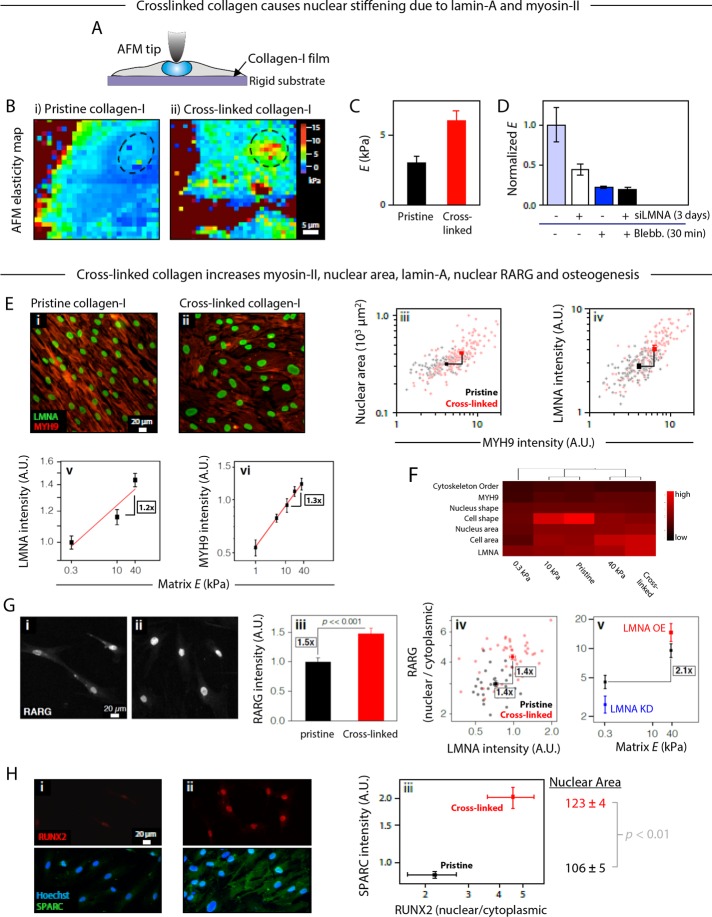
FIGURE 4:
Influence of matrix mechanics on osteogenic pathways: effect of collagen cross-linking on nuclear elasticity and protein expression. (A) AFM was used to probe the stiffness profiles of MSCs cultured on a rigid substrate, thus allowing an in situ readout of cellular elasticity without having to deconvolute effects of substrate deformation. (B) Force–volume mode elasticity maps of living cells cultured for 6 d on (i) pristine and (ii) cross-linked collagen-1 films, showing that matrix cross-linking caused a twofold increase in the Young's modulus of the nuclear region (dashed circles). (C) Young's moduli obtained from force–indentation curves at the position of the nucleus, averaged from ∼60 curves/cell and 7–13 individual/sample, cultured on pristine of cross-linked collagen films. (D) Relative contributions to the normalized stiffness of the nuclear region from the nuclear lamina and cortical tension in the actomyosin network can be appreciated by treatments with small interfering LMNA (siLMNA) and blebbistatin, respectively (averaged from ∼60 force–indentation curves measured at different locations within the nuclear region of four to seven cells cultured on plastic). (E) MSCs cultured for 2 wk on cross-linked collagen-1 films have 1.5-fold higher levels of LMNA. Immunofluorescence images of LMNA (green) and myosin-IIA (MYH9, red) on (i) pristine and (ii) cross-linked collagen-I films. Quantitative image analysis showed that (iii) increased levels of MYH9 correlated with greater nuclear spread area and (iv) higher levels of LMNA (n > 115 cells; significantly different in both dimensions, p < 0.01). (v, vi) LMNA and MYH9 levels in MSCSs on gels shown for comparison. (F) Heat map summary and hierarchical clustering of cellular and nuclear parameters, as well as protein expression, on 0.3-, 10-, and 40-kPa gels and both pristine and cross-linked collagen films from various experiments. The dendogram indicates that cells on 10-kPa gels and pristine films cluster together, as do cells on 40-kPa gels and cross-linked films, whereas cells on soft, 0.3-kPa gels are distinct from the others. (v) LMNA intensity on gels. (vi) MYH9 intensity on gels. (G) Comparison of the cellular location of transcription factor RARG in MSCs cultured on (i) pristine and (ii) cross-linked collagen-I films. (iii) A quantification of RARG levels showed a significant increase on cross-linked substrate (n > 50 cells). (iv) A cell-by-cell analysis showed that cross-linked collagen films favor higher LMNA and a correlated increase in the nuclear-to-cytoplasmic ratio of RARG (n > 30 cells; significantly different in both dimensions, p < 0.01). (v) Average nuclear-to-cytoplasmic ratio of RARG on gels. (H) RUNX2 and SPARC were also compared in MSCs cultured on (i) pristine and (ii) cross-linked collagen-I films. Quantification of the images (iii, correlating results from two separate experiments) showed that cross-linked substrates favored a greater nuclear localization of transcription factor RUNX2 and a greater expression of osteogenic marker SPARC. Nuclear spread area is also highest on cross-linked films, as predicted from the fits of Figure 3D i. All experiments, n ≥ 3 (mean + SEM).
To assess the contribution of lamin-A to nuclear stiffness—which could be direct or indirect through other partners and pathways—we knocked lamin-A down by ∼50% and found a proportional decrease in apparent nuclear stiffness (Figure 4D). This is consistent with direct contributions to stiffness of lamin-A in MSCs and other cell types as studied by micropipette aspiration, in which measurements are made after cell detachment and actin depolymerization within 1–2 h (Harada et al., 2014). However, because it is also known that inhibiting the contractile actomyosin network with blebbistatin decreases “nuclear tension” through a decrease in both nuclear area and lamin-A levels in MSCs within hours, inhibition of nuclear tension should also make the nucleus measurably softer (analogous to a tensed rope being laterally stiffer until the rope is cut). An approximately fourfold reduction in the apparent nuclear stiffness with blebbistatin is indeed measured and is consistent with recent AFM studies comparing isolated nuclei to nuclei in intact cells (Liu et al., 2014). Blebbistatin plus lamin-A knockdown combined did not further decrease the apparent nuclear stiffness in the MSCs, probably because 1) blebbistatin alone tends to quickly decrease lamin-A levels (Buxboim et al., 2014), and 2) lamin-A knockdown alone reduces the levels of myosin-II in MSCs over days through the serum response factor (SRF) pathway (Ho et al., 2013; Swift et al., 2013b; Buxboim et al., 2014; Talwar et al., 2014). In other words, endogenous lamin-A, contractility, and nuclear stiffness are all mechanistically linked—at least in these cells.
MSCs expressing ectopic green fluorescent protein (GFP)–lamin-A (Supplemental Figure S3A) had an apparent nuclear stiffness (on cross-linked nanofilms) that increased with GFP-lamin-A level. To eliminate contributions of the cytoskeleton, MSCs were detached, the actomyosin cytoskeleton was depolymerized with latrunculin, and micropipette aspiration was used to measure nuclear stiffness in suspended cells. The results demonstrate increased nuclear stiffness with GFP–lamin-A independent of cytoskeleton (Supplemental Figure S3B). Nonetheless, time-dependent changes in apparent nuclear stiffness over 2 wk in culture on cross-linked nanofilms (Supplemental Figure S3C) are likely a result of changes in both lamin-A levels and actomyosin tension.
For MSCs cultured on pristine or cross-linked films, immunofluorescence analyses of lamin-A nuclear area and myosin-IIA in single cells at 2 wk (Figure 4E, i and ii) generally revealed that LMNA correlated with increased nuclear spreading and levels of MYH9 (Figure 4E, iii and iv). Of importance, collagen cross-linking caused a 1.5-fold average increase in LMNA, which is consistent with observations of MSCs on soft versus stiff gels (Figure 4E, v and vi; Swift et al., 2013b; Buxboim et al., 2014). Scatter plots for all of these single-cell analyses always show that some cells on the cross-linked films fall within the response envelopes of cells on pristine films, so that this shared subpopulation of nonresponding cells at least provides a common basis for extrapolation of the trend. A heat map of quantified morphologies and protein levels in MSCs on the nanofilms and gels of varied stiffness (Figure 4F) clusters together the cell phenotype on pristine films with that on 10-kPa gels and separately clusters together the cell phenotype on cross-linked films with that on 40-kPa gels, whereas cell phenotypes on soft gels are most distinct. Compared to similar analyses of transcriptomes, this direct alignment of cell phenotypes is likelier to reflect mechanosensitive processes such as protein degradation that sometimes decouple from transcript levels (Dingal et al., 2015).
Immunostaining for the transcription factor RARG, which generally correlates with lamin-A in tissues (Figure 2C) and can in principle regulate LMNA transcription (Swift et al., 2013b), showed that the total amount of RARG increased on cross-linked films after just 2 d in culture (Figure 4G, i–iii). RARG's nuclear-to-cytoplasmic ratio in cells on nanofilms also correlated with lamin-A intensity in a cell-by-cell analysis, which is in good agreement with similar trends for cells on gels (Figure 4G, iv and v). Mechanistic links are supported here later by short-time kinetic studies that use soluble RA ligand.
Based on the surprisingly general association between collagen cross-linking and levels of key osteogenic factors (Figure 2C), MSCs on the two types of nanofilms were assessed for osteoinduction without addition of any osteogenic soluble factors. RUNX2 is an early osteogenic transcription factor required for osteogenesis (Choi et al., 2001); RUNX2 was mostly nuclear after 1 wk in culture and greater than twofold more so on cross-linked nanofilms (Figure 4H). The results are consistent with matrix elasticity–directed lineage specification on stiff gels even in the absence of “osteoinduction media” (Engler et al., 2006; Benoit et al., 2008; Chen et al., 2010). SPARC (or osteonectin) is a late marker of osteogenesis and is a bone glycoprotein secreted by osteoblasts to initiate mineralization (Termine et al., 1981); SPARC was also greater than twofold higher after 2 wk on cross-linked films. Nuclei in the more osteogenic state were more spread (p < 0.01), which once again suggests higher cytoskeletal tension on the stiffer matrix. The findings thus suggest that 1) the stiffness of cross-linked nanofilms is osteoinductive, and 2) stiffness sensing might be amplified by coupling to soluble factor regulation of the increased nuclear RARG.
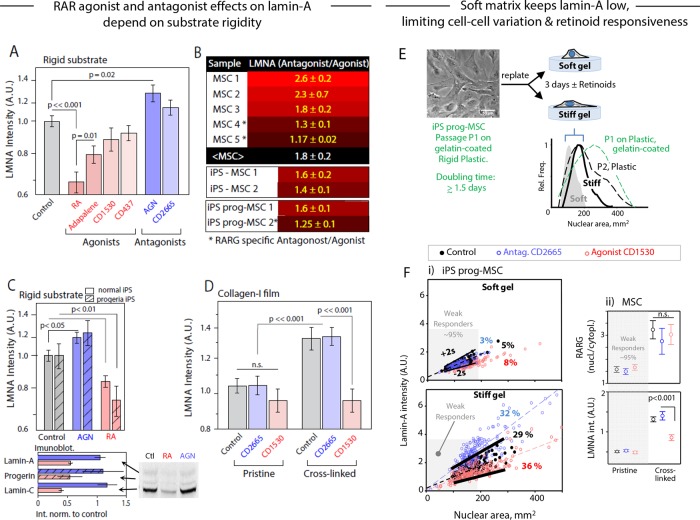
RARG antagonist and agonist respectively increase/maintain or decrease LMNA and progerin only on stiff substrates
RA signaling pathways affect many developmental and homeostatic processes. We previously found that the pan-RAR antagonist AGN (AGN193109; Supplemental Figure S4A) enhances osteogenesis of MSCs cultured in osteoinduction medium (OIM; Swift et al., 2013b). RARG-specific agonists slow ectopic bone growth in mouse models (Shimono et al., 2012), but antagonists have been unexplored. Here we tested antagonists or agonists that are more specific to RARG (Supplemental Figure S4A), and for a wider range of MSCs, we first measured the effects on LMNA levels. Whereas agonists tended to decrease LMNA, antagonists tended to increase LMNA on standard rigid culture plastic (Figure 5A). To clarify the trend on various stiff or rigid substrates, we tabulated the (antagonist/agonist) effect on LMNA across primary MSCs for various drug pairs and found 1.8 ± 0.2 for the mean ratio (± SEM; Figure 5B and Supplemental Figure S4B). Donor variability of MSCs is at least one source of variation.
FIGURE 5:
Influence of soluble factors on RA pathway implicated in osteogenesis: convergence of mechanochemical effects. (A) MSCs cultured on plastic with drugs that perturb the RA pathway (control is ethanol/dimethyl sulfoxide vehicle only; drug treatments at 1 µM, except adapalene and CD437 at 0.1 µM; see Supplemental Figure S4A for summary of drug properties). LMNA was quantified by immunostaining after 4 d. (B) Although the magnitude of response varied across MSCs from different donors, RA pathway antagonists consistently increased LMNA level with respect to the agonist on a range of stiff substrates (rigid plastic, stiff polyacrylamide gels, collagen thin films; see Supplemental Figure S4B for full table); iPS-derived MSCs and progeria patient–derived iPS-MSCs gave similar response (bottom of the table). (C) Antagonist/agonist effects on LMNA levels from immunofluorescence of iPS-MSCs and progeria patient–derived iPS-MSCs relative to control. The immunoblots (bottom) showed that the progerin from the disease-causing allele was affected as much as the normal lamin-A spliceforms. (D) LMNA was quantified by immunofluorescence in MSCs treated for 3 d with RARG-specific RA pathway antagonist CD2665 or agonist CD1530 while being cultured on pristine or cross-linked collagen-I films. Consistent with earlier reports (Swift et al., 2013b), a significant differential response between agonist and antagonist is apparent only on a “stiff” (cross-linked) substrate. (E) (i) The spread area of nuclei in iPS-progeria–derived MSCs depends on matrix stiffness and is reversible. Early passage, P1, MSCs spread less as P2 on soft gels than on stiff gels or plastic. (F) Nuclear area and lamin-A in iPS-progeria–derived MSCs on soft gels of 2 kPa shows that 95% of cells are within ±2σ of untreated cells even after treatment with RA agonist/antagonist. These cells are “weak responders” to retinoids, and the same range of small nuclear areas identifies weak responders to retinoids on stiff gels of 50 kPa. However, about one-third of cells are mechanosensitive to stiffness, with elevated lamin-A levels that also change in response to retinoids. (ii) Nuclear RARG in bone marrow–derived MSCs on pristine and cross-linked films increases (for responding cells) with matrix stiffness, but retinoids have no effect on RARG, even though lamin-A tends to decrease with agonist and increase with antagonist.
The iPSCs can be a more uniform source of MSCs that turn on expression of lamin-A with loss of pluripotency (Zhang et al., 2011; Talwar et al., 2013). The iPSC-MSCs also have no memory of the many factors in a bone marrow niche. Application of retinoids to iPSC-MSCs gave (antagonist/agonist) effects on LMNA levels that were statistically the same as for primary bone marrow MSCs (Figure 5B). Of importance, progeria patient–derived iPSC-MSCs showed similar effects on lamin-A levels with both RARG-specific and -nonspecific drugs (Figure 5C and Supplemental Figure S5F). Although progerin is a mutated form of lamin-A protein that causes accelerated aging, expression of the progerin allele should be unaffected. Immunoblots showed the drugs affect progerin from the disease-causing allele as much as the normal lamin-A splice-forms (Figure 5C, bottom, and Supplemental Figure S5E). Retinoid effects are strong in early passages when cultured on rigid substrates, where the nucleus is well spread (Supplemental Figure S5C). Agonist treatment also resulted in an ∼40% increase of nuclei with irregular shapes and showed LMNA enrichment in high-curvature regions (tips; Supplemental Figure S5D). Previous studies with other cell types showed that increased progerin results in misshapen nuclei (Goldman et al., 2004), but cytoskeletal forces in the MSCs studied here could have different effects on nuclei. For all of the different MSCs tested, the findings are nonetheless consistent with retinoids contributing to transcriptional control of LMNA.
To test the functional consequences of the low nuclear RARG seen in MSCs on pristine collagen nanofilms (Figure 4F), we compared the RARG-specific antagonist CD2665 with the RARG-specific agonist CD1530 in terms of effects on LMNA in MSCs on the two types of nanofilms. The cross-linked nanofilm produced the expected LMNA ratio for antagonist/agonist, but the pristine nanofilm, which was soft (Figure 3, D and E), gave no significant retinoid effects (Figure 5D). Lamin-A levels were nonetheless highest in MSCs on cross-linked nanofilms, consistent with Figure 4E. For insight into the kinetics of the effect of the RARG-specific antagonist CD2665, we treated MSCs on a rigid substrate with drug and imaged them. Within 40 min, nuclear localization of RARG increased (by ∼15%; p = 0.05) and remained high, whereas LMNA required 4 h to be significantly higher (Supplemental Figure S4C). Taken together, these findings suggest fast-acting cooperation between soluble factor and mechanosensitive pathways.
To assess the retinoid responsiveness in cooperation with matrix stiffness, we examined how lamin-A level depends on nuclear area when treated with RA agonist/antagonist. First, we established the reversibility of nuclear spreading: iPSCs were induced to differentiate into MSCs with well-spread cells and nuclei on gelatin-coated plastic (which is rigid with E > 100 kPa based on AFM measurements), but replating onto soft gels (2 kPa) caused a decrease in both nuclear area and its variation (Figure 5E). Parallel analyses of distributions for Lamin-A as well as nuclear area revealed a “weak-responder” subpopulation of cells on stiff gels that respond like 95% of cells on soft gels. About one-third of cells sense the stiffness and spread with high lamin-A, and retinoids affect an equal fraction (32–36%) in regulating lamin-A levels (Figure 5Fi). The same proved true for bone marrow–derived MSCs on pristine and cross-linked films. Of importance, retinoids have no effect on nuclear translocation of RARG in cells on either film, even though the cross-linked matrix drives more RARG into the nucleus and lamin-A decreases with agonist while increasing slightly with antagonist (Figure 5Fii).
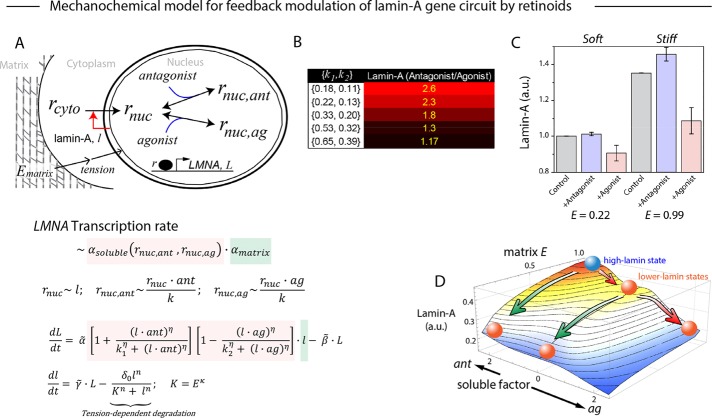
A parsimonious dynamical model of the mechanochemical effects on the LMNA gene circuit was developed to fit the data (Figure 6A). We incorporated the finding that lamin-A protein is stabilized by cytoskeletal stresses on stiff matrices (Dingal and Discher, 2015). Similar models have been developed for cytoskeletal regulation of SRF genes (Jain et al., 2013). Soluble factor effects on RARG translocation for LMNA transcription were incorporated (Figure 6A), and variation of antagonist/agonist effects on lamin-A levels (e.g., cell-to-cell variation) were emulated by varying the relative strength of RARG–antagonist/agonist binding to the LMNA promoter (Figures 5B and 6B). In particular, increased binding sensitivities (lower k’s) increased the modulation by soluble factors of LMNA. This matrix-modulated noise analysis appears novel and might imply that for individual cells that start more stem-like and less osteogenic, soluble-factor induction of LMNA via RARG is most effective. A matrix elasticity–dependent nuclear localization of RARG (rnuc) was also necessary to capture soluble-factor effects on lamin-A levels (Figures 5C and 6C). The model predicted not only the orthogonality of purely mechanical and purely chemical pathways but also that the combination synergizes to maximally regulate levels of lamin-A protein (Figure 6D).
FIGURE 6:
Waddington-inspired model of mechanochemical effects on lamin-A levels. (A) Lamin-A (l) levels increase with matrix elasticity (Ematrix) and influence cytoplasmic RARG (rcyto) shuttling into the nucleus (rnuc), where its binding on LMNA (L) promoter as a repressor can be enhanced or abrogated by soluble agonist (ag) or antagonist (ant). Transcription rate of LMNA is a function of both soluble (αsoluble) and matrix (αmatrix) factors. Soluble effects are, in turn, modulated by the levels of RARG-antagonist (rnuc,ant) and RARG-agonist (rnuc,ag) complexes, whose dissociation constants are represented by k1 and k2, respectively. Building from our previous model, in which lamin-A's tension-inhibited degradation, K, is key to its scaling with matrix elasticity, E, we extended the model to capture the convergence of mechanical and chemical effects on lamin-A. Model parameters were specified by recapitulating experimental results in Figure 5, B and D: 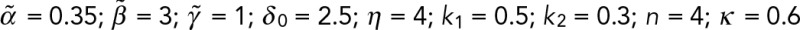 . (B) Sensitivity analysis of varying binding strengths of RARG antagonist (k1) or agonist (k2) complexes to lamin-A expression response (E = 0.99, {ant, ag} = {1,0} or {0,1}). (C) For pristine (relative E = 0.22) and cross-linked (relative E = 0.99) films, antagonist (ant) and agonist (ag) values were set to 1.0. Error bars are SDs derived from simulating 100 cells with k1 and k2 randomly generated as values within the ranges [0.4, 0.6] and [0.2, 0.4], respectively. (D) The model predicts two paths from a high-lamin state (blue sphere) to low lamin-A states (red spheres) by reducing matrix E (green arrows) or adding soluble agonist (red arrows). All experiments, n ≥ 3 (mean + SEM).
. (B) Sensitivity analysis of varying binding strengths of RARG antagonist (k1) or agonist (k2) complexes to lamin-A expression response (E = 0.99, {ant, ag} = {1,0} or {0,1}). (C) For pristine (relative E = 0.22) and cross-linked (relative E = 0.99) films, antagonist (ant) and agonist (ag) values were set to 1.0. Error bars are SDs derived from simulating 100 cells with k1 and k2 randomly generated as values within the ranges [0.4, 0.6] and [0.2, 0.4], respectively. (D) The model predicts two paths from a high-lamin state (blue sphere) to low lamin-A states (red spheres) by reducing matrix E (green arrows) or adding soluble agonist (red arrows). All experiments, n ≥ 3 (mean + SEM).
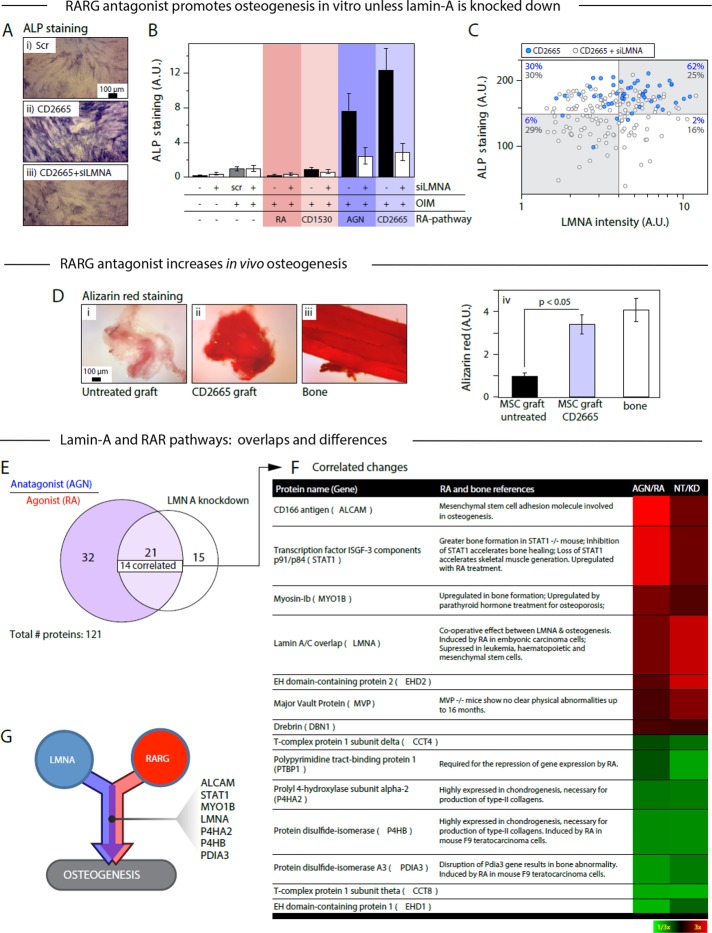
RARG antagonist and agonist respectively increase and decrease osteogenesis in vitro and in vivo
To assess functional effects of retinoids on osteogenesis, we assayed ALP activity as a first readout for osteoinduced MSCs cultured with OIM on rigid substrates. The RARG-specific antagonist CD2665 showed the greatest effect in enhancing ALP staining of cultures, by ∼12-fold relative to osteoinduced MSC controls not treated with retinoids. CD2665s effect was also 50% greater than that of the pan-RAR antagonist AGN. Of importance, LMNA knockdown decreased ALP nearly to control levels for the antagonists (Figure 7, A and B). Agonists that were either RARG-specific (CD1530) or pan-RAR (RA) tended to decrease ALP but with little effect of LMNA knockdown. With the RARG-specific antagonist CD2665, single-cell analyses show that LMNAhigh cells are sixfold more likely to also be ALPhigh than LMNAlow cells, and the supermajority of cells (62%) are both ALPhigh and LMNAhigh (Figure 7C). Knockdown of LMNA increases the ALPlow subpopulations by greater than fivefold despite the presence of antagonist. Although retinoids can affect many pathways, given their demonstrated influence on lamin-A levels (e.g., Figure 5; via promoter-based transcriptional regulation (Okumura et al., 2004)), and given the evidence that nuclear accumulation of RARG is favored by LMNAhigh (e.g., Figure 4G), it is fully consistent to find that differentiation also exhibits synergy between RARG-specific soluble factors and matrix rigidity.
FIGURE 7:
Proteomic comparison of the effects of LMNA knockdown and drug perturbations to the RA pathway allows identification of factors common to both pathways. (A) MSCs were cultured on plastic for 1 wk with combinations of OIM, siLMNA, and drug perturbations to the RA pathway. (i) ALP staining of scrambled RNA and OIM. (ii) RARG-specific antagonists CD2665 and OIM. (iii) siLMNA, CD2665, and OIM. (B) ALP activity is enhanced in the presence of RA antagonists, but this effect is abrogated by LMNA knockdown. (C) A cell-by-cell analysis of LMNA level correlated with local ALP staining in MSCs cultured on rigid substrate with OIM and CD2665, with or without siLMNA, showed that higher LMNA levels positively correlate with greater osteogenesis (Supplemental Figure S5A). The percentages indicate the number of points (blue, without siLMNA; gray, with siLMNA) in quadrants defined by the means of the siLMNA points. (D) Antagonist to RARG also increased osteogenesis in vivo: MSCs were engrafted into mouse flank, excised after 4 wk, and stained with alizarin red, an indicator of osteogenic calcification: (i) untreated MSC graft; (ii) CD2665 pretreated MSC graft; (iii) mouse femur; (iv) quantification of staining. These experiments done in duplicate suggest that CD2665 pretreatment increased the bone-like character of the grafted MSCs (Supplemental Figure S5, B and D). (E) MSCs were cultured on plastic for 8 d with drug perturbation to the RA pathway or subjected to LMNA knockdown. Cells were analyzed by MS proteomics: of 121 proteins quantified (with more than three peptides per protein), 53 varied by >4/3-fold in the RA-perturbation experiment, and 36 varied by the same degree in the siLMNA experiment (Supplemental Figure S6A). Twenty-one proteins varied in both experiments, and of these, 14 were positively correlated. (F) Table of correlated protein changes with a heat map representation of fold change in RA pathway and siLMNA experiments. See Supplemental Figure S7 for references. (G) Representation of proteins involved with overlapping pathways to osteogenesis. Unless indicated, n ≥ 3 (mean + SEM).
To provide further evidence that the RARG-specific antagonist CD2665 promotes osteogenesis, MSCs were pretreated with drug (or not) for 3 d, implanted into mouse flank for 2–3 mo per Figure 2A, and then analyzed for calcification. With live mice, fluorescent bisphosphonate was injected and imaged, revealing a bone-like intensity for CD2665-treated xenografts that was significantly greater than controls (Supplemental Figure S6, B and C). Xenograft tissue was removed and stained with alizarin red for calcification. Antagonist gave a calcification signal similar to bone, which is threefold to fourfold greater than xenografts from either untreated control or agonist pretreated MSCs (Figure 7D). ALP staining showed similar trends for the xenografts (Supplemental Figure S6E). The RARG-specific antagonist thus promotes osteogenesis in vitro and in vivo.
Convergent overlap in synergistic differentiation
Although cell-to-cell variation can be large (Figure 7C), robustness of differentiation in noisy systems can be achieved through parallel systems of feedback (Ahrends et al., 2014). Proteomic changes were therefore compared between MSCs treated with AGN versus RA, as characteristic of the soluble factor input, and control versus LMNA knockdown. Based on detection of three or more tryptic peptides per protein, 21 proteins (of 121 quantified) varied by >1.33-fold in both experiments (Supplemental Figure S7A), and most exhibited correlated responses to both perturbations (Figure 7E). Given the wide-ranging studies of retinoids that rarely if ever mention lamin-A, it seems unsurprising that most pathways regulated by retinoids differ from those affected by lamin-A; this makes their interplay in differentiation that much more biologically significant. Gene ontology and literature analyses of the 21 overlapping proteins nonetheless identified a number of molecules relevant to synergy in osteogenic processes (Figure 7F), particularly STAT1, MYO1B, and PDIA3 (Miyaishi et al., 1998; Balmer and Blomhoff, 2002; Xiao et al., 2004; Tajima et al., 2010; Wang et al., 2010; Roosa et al., 2011; Gao et al., 2012). Further comparison to transcriptional changes after LMNA knockdown suggested additional overlap candidates while adding confidence to common factors STAT1, ALCAM, EHD2, MVP, and P4HA2 (Figure 7G and Supplemental Figure S7B).
DISCUSSION
Collagen cross-linking, stiffer tissues, and stiffer nucleus
A cell might respond to the rigidity of a single matrix fibril that integrins bind (Watt and Huck, 2013), or it might respond to collective matrix properties at a larger scale. Stress-bearing bone, cartilage, and muscle must all have sufficient strength and stiffness at a macroscopic scale to sustain the large forces of tissue function (e.g., walking). However, the microenvironment with which bone-making osteoblasts interact is collagen rich and not calcified (Sodek and McKee, 2000), which should make this osteoid much softer than the large-scale properties of rigid bone. MSCs that enter such an osteogenic niche from marrow (such as perivascular niches; Park et al., 2012) transit from a very soft marrow niche to a stiff but thin collagenous niche, for which the novel nanoscale measurements here indicate is softer than bone (Figure 1). Osteoid is reasonably approximated in terms of cell-perceived stiffness (Figure 3) by a cross-linked collagen-I nanofilm, which is more osteogenic for MSCs than a pristine collagen-I nanofilm (Figure 4). Broader significance is suggested by meta-analysis of transcriptomes across many tissues (excluding bone; Figure 2), with potential relationships emerging between cross-linked collagen, lamin-A, which confers nuclear stiffness, and RARG, which can in principle regulate lamin-A transcription (Swift et al., 2013b) and even osteoinduction. The latter might lend insight into ectopic calcification that is sometimes seen after injury coincident with scars having an abundance of collagen that is highly cross-linked (Shimono et al., 2012). However, the response of lamin-A to matrix rigidity appears more general and can occur even within hours (Supplemental Figure S4; Buxboim et al., 2014), but whether RARG and downstream pathways are independent or synergize for differentiation is key to understanding signals in a niche.
With cross-linked collagen-I, if a cell adheres to and pulls on even one fibril, bending of that fibril will also require bending of adjacent fibers (Figure 3), which means that the cell should feel a stiff film. With synthetic gels that are also modified with adhesive ligands such as collagen, molecular-scale mechanisms of force transmission remain unclear (Watt and Huck, 2013). Nonetheless, gel stiffness generally drives cell spreading, which requires actomyosin stress fiber formation (Discher et al., 2005), and the MSC nucleus also becomes more spread and flattened, with lamin-A levels increasing while B-type lamins are unchanged (Supplemental Figure S2, F and G). Cross-linked collagen-I films and stiff gels cause similar MSC responses (Figure 3), but only the cross-linked nanofilms on rigid mica permit probing of nuclear stiffness (as opposed to indenting nuclei into gels beneath a cell), helping to show that nuclear stiffening parallels matrix stiffening (Figure 4). Nuclear stiffening's dependence on lamin-A and actomyosin is consistent with the pathway for lamin-A accumulation in MSCs on stiff substrates: actomyosin tension opposes lamin-A phosphorylation and degradation that occurs in cells on soft gels (Buxboim et al., 2014). Downstream pathways are reasonably clear because we previously showed (Swift et al., 2013b) that the lamin-A–binding protein SUN2 coimmunoprecipitates with RARG and that overexpressed SUN2 decreases both nuclear RARG and lamin-A. We can therefore infer that high lamin-A levels favor nuclear retention of SUN2 and RARG, which primes the latter for transcriptional control of LMNA. Consistent with these aspects of mechanism worked out with cells on gels, cross-linked nanofilms here indeed increase nuclear RARG relative to MSCs on pristine films (Figure 4), and addition of RARG-specific antagonist increases nuclear accumulation of RARG and lamin-A levels within hours (Supplemental Figure S4). Whereas pan-RAR agonists and antagonists suggest roles for RARs (Swift et al., 2013b), RARG-specific agonists and antagonists provide functional evidence for this isoform distinct from RARA and RARB (Figure 5 and Supplemental Figure S4). Our calculations for the mechanochemical gene circuit thus couple matrix elasticity regulation of lamin-A protein to effects of RARG soluble factor on lamin-A expression. Of importance, RARG antagonist/agonists are ineffective on MSCs on soft matrix according to both experiments and calculations (Figure 5).
Implications for progerin and aging
Microenvironments trigger and direct stem cell differentiation, but microenvironments can change in aging, and so can lamin-A. For example, collagen-I–rich skin (Figure 2A) stiffens twofold to threefold in human aging (Diridollou, 2001), and progerin, in addition to normal lamin-A, has been detected in skin biopsies and fibroblasts of normal donors >80 yr of age (Dahl et al., 2006; McClintock et al., 2007). The potential rejuvenating effects of RA on aging tissues such as skin has many complexities (Mukherjee et al., 2006), and the results here for progerin, as well as normal lamin-A (Figure 5B and Supplemental Figure S5), suggest additional complexity because such soluble factors are generally applied to cells adhering to matrix or another cell that can influence cell responses as extreme as differentiation. Furthermore, RAR antagonists might naturally derive from vitamin A (Eroglu et al., 2012), as is the case for RA, and so the antagonist/agonist balance or imbalance in a given microenvironment or niche could contribute to levels of both normal lamin-A and progerin. Levels can have a dramatic effect on health: homozygous lamin-A–knockout mice all die 3 wk postpartum with major heart and cardiovascular defects (similar to progerin mice that die), whereas heterozygotes maintain normal heart function at least 20-fold longer (Kubben et al., 2011). Beyond retinoids and lamins, responses of cells on rigid coverslip glass or tissue culture plastic to other drugs that converge on mechanosensitive pathways could be different from those of the same cells on soft, tissue-like matrix.
Translatable potential versus matrix-associated growth factors
Matrix-associated growth factors such as transforming growth factor β superfamily members have potent effects on stem cells, including effects of bone morphogenetic protein 2 (BMP 2) on bone formation (e.g., Hayashi et al., 2009). However, the release of such matrix-bound factors depends in part on matrix mechanics and cell contractility (Wipff et al., 2007), which of course tie such factors to matrix pathways that are distinct from purely soluble factors such as retinoids (Figure 1A). Implants that are simply loaded with recombinant human BMP cause ectopic bone (Carragee et al., 2011). RARG antagonist pretreatment of human bone marrow MSCs leads to human bone formation in vivo even without addition of BMPs (Figure 7D) and could prove a useful alternative for bone tissue engineering. Of importance, whereas matrix inputs might only affect cells while they are in contact with matrix (Schellenberg et al., 2014), short periods of soluble factor pretreatment of stem cells can have long-term effects such as in vivo osteogenesis over many weeks. Retinoids can certainly drive epigenetic programs (Gudas, 2013), and matrix mechanics could exert similar effects, especially given the mechanosensitivity of lamin-A.
MATERIALS AND METHODS
AFM on primary tissue and cells on films
Tissue samples or mica disks with cultured cells were attached to a glass dish fully immersed in medium at 37°C and indented with soft cantilevers with nominal spring constants of 0.01 N/m when probing bone marrow or 0.05 N/m for osteoid and cells. Before each experiment, cantilevers were calibrated using a thermal fluctuations method. The distribution of Young's modulus of bone tissues was evaluated from measurements at multiple locations in tibia samples from six different mice. Silverman's test (Silverman, 1981) for multimodality in R package “silvermantest’’ (www.uni-marburg.de/fb12/kooperationen/stoch/forschung/rpackages) was used with a null hypothesis that the logarithm of the data has at most two modes. The p value of the test was <0.01, which provides strong statistical evidence for at least three modes. The Sneddon modification to the Hertz model for a conical tip was used to calculate the apparent Young's modulus, assuming indentations to an isotropic, homogeneous material. The depth of indentations was determined using the procedure in Buxboim et al. (2010); it is between 400 nm and 1 µm, and the fitting range was obtained by minimizing the fitting error assuming a Poisson ratio of 0.5. All measurements were performed within hours of mouse killing in order to preserve the in vivo properties of the tissue. Cells on collagen films were probed in force–volume mode with a resolution of 128 × 128 indentation curves per frame, with a scanning frequency of 0.2 Hz. Topographical images of fixed cells were taken in contact mode.
Preparation of thin collagen films
Rat-tail collagen solution (CB354249; Corning; 200 µl at 1 µg/ml in 50 mM glycine buffer, 200 mM KCl, pH 9.2) was deposited on freshly cleaved mica disks at room temperature for self-assembly. After 30 min, liquid was gently aspirated, and disks were washed with Dulbecco's phosphate buffered saline (DPBS). The collagen films were cross-linked with tissue transglutaminase (T5398; Sigma-Aldrich; concentration 30 µg/ml in 50 mM Tris buffer, 5 mM CaCl2, and 2 mM dithiothreitol at pH 7.4). The films were immersed in the solution and agitated for 4 h at 37°C before washing with DPBS.
AFM of thin films
Collagen films were imaged in DPBS at room temperature in tapping mode with driving frequency close to the resonance frequency of the cantilever. Fibril deformation was performed in a direction perpendicular to the fibril orientation in contact mode with predefined force and trajectory. After scratching, films were reimaged in tapping mode.
Supplementary Material
Acknowledgments
We thank the Wistar Institute Proteomics Core for MS support, D. Gilbert (Florida State University) for GFP–lamin-A, and A. Miyawaki (RIKEN) for the genetically encoded probe for RA, GEPRA. We were supported by National Institutes of Health/National Cancer Institute PSOC Award U54 CA193417, National Heart Lung and Blood Institute Awards R01 HL124106 and R21 HL128187, a National Science Foundation Materials Science and Engineering Center grant to the University of Pennsylvania, the US–Israel Binational Science Foundation, and Charles Kaufman Foundation Award KA2015-79197. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
Abbreviations used:
- AFM
atomic force microscope
- ALP
alkaline phosphatase
- E
Young’s modulus of elasticity or stiffness
- GFP
green fluorescent protein
- iPSC
induced pluripotent stem cells
- MS
mass spectrometry
- MSC
mesenchymal stem cell
- OIM
osteoinduction media
- RA
retinoic acid
- RAR
retinoic acid receptor.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-01-0010) on May 31, 2017.
References
- Ahrends R, Ota A, Kovary KM, Kudo T, Park BO, Teruel MN. Controlling low rates of cell differentiation through noise and ultrahigh feedback. Science. 2014;344:1384–1389. doi: 10.1126/science.1252079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B, Daulton TL, Genin GM, Lipner J, Pasteris JD, Wopenka B, Thomopoulos S. The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen-mineral structure. J R Soc Interface. 2012;9:1774–1786. doi: 10.1098/rsif.2011.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jallad HF, Nakano Y, Chen JLY, McMillan E, Lefebvre C, Kaartinen MT. Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MOT3-E1 osteoblast cultures. Matrix Biol. 2006;25:135–148. doi: 10.1016/j.matbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger JM, Foeger N, Kill IR, Herrmann H. The nuclear lamina—both a structural framework and a platform for genome organization. FEBS J. 2007;274:1354–1361. doi: 10.1111/j.1742-4658.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- Buxboim A, Rajagopal K, Brown AEX, Discher DE. How deeply cells feel: methods for thin gels. J Phys Condens Matter. 2010;22, 194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxboim A, Swift J, Irianto J, Athirasala A, Kao Y-RC, Spinler KR, Dingal PCDP, Discher DE. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Chen WLK, Likhitpanichkul M, Ho A, Simmons CA. Integration of statistical modeling and high-content microscopy to systematically investigate cell-substrate interactions. Biomaterials. 2010;31:2489–2497. doi: 10.1016/j.biomaterials.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Choi JY, Pratap J, Javed A, Zaidi SK, Xing LP, Balint E, Dalamangas S, Boyce B, van Wijnen AJ, Lian JB, et al. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci USA. 2001;98:8650–8655. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros DA, Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Creating ultrathin nanoscopic collagen matrices for biological and biotechnological applications. Small. 2007;3:956–963. doi: 10.1002/smll.200600598. [DOI] [PubMed] [Google Scholar]
- Cox TR, Bird D, Baker AM, Barker HE, Ho MWY, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, adipocytes. J Biomech. 2008;41:454–464. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingal P, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingal P, Discher DE. Systems mechano-biology: tension-inhibited protein turnover is sufficient to physically control gene circuits. Biophys J. 2015;108:365A–366A. doi: 10.1016/j.bpj.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diridollou S. Mechanical properties of the skin under suction. Skin Res Technol. 2001;7:127–127. doi: 10.1034/j.1600-0846.2001.70212.x. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Jr, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287:15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J Mol Biol. 2007;372:594–607. doi: 10.1016/j.jmb.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li YF, Guo X, Wu ZG, Zhang W. Loss of STAT1 in bone marrow-derived cells accelerates skeletal muscle regeneration. PLoS One. 2012;7:9. doi: 10.1371/journal.pone.0037656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol. 2013;24:701–705. doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann Biomed Eng. 2005;33:1312–1318. doi: 10.1007/s10439-005-4479-7. [DOI] [PubMed] [Google Scholar]
- Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal P, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C, Hasegawa U, Saita Y, Hemmi H, Hayata T, Nakashima K, Ezura Y, Amagasa T, Akiyoshi K, Noda M. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J Cell Physiol. 2009;220:1–7. doi: 10.1002/jcp.21760. [DOI] [PubMed] [Google Scholar]
- Heath DJ, Downes S, Verderio E, Griffin M. Characterization of tissue transglutaminase in human osteoblast-like cells. J Bone Miner Res. 2001;16:1477–1485. doi: 10.1359/jbmr.2001.16.8.1477. [DOI] [PubMed] [Google Scholar]
- Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci USA. 2013;110:11349–11354. doi: 10.1073/pnas.1300801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen MT, El-Maadawy S, Rasanen NH, McKee MD. Tissue transglutaminase and its substrates in bone. J Bone Miner Res. 2002;17:2161–2173. doi: 10.1359/jbmr.2002.17.12.2161. [DOI] [PubMed] [Google Scholar]
- Katz EP, Li S. Structure and function of bone collagen fibrils. J Mol Biol. 1973;80:1–5. doi: 10.1016/0022-2836(73)90230-1. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1(+) progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, Voncken JW, Konings G, van Weeghel M, van den Hoogenhof MMG, Gijbels M, van Erk A, Schoonderwoerd K, van den Bosch B, Dahlmans V, et al. Post-natal myogenic and adipogenic developmental Defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207. doi: 10.4161/nucl.2.3.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HJ, Wen J, Xiao Y, Liu J, Hopyan S, Radisic M, Simmons CA, Sun Y. In situ mechanical characterization of the cell nucleus by atomic force microscopy. ACS Nano. 2014;8:3821–3828. doi: 10.1021/nn500553z. [DOI] [PubMed] [Google Scholar]
- Lovett DB, Shekhar N, Nickerson JA, Roux KJ, Lele TP. Modulation of nuclear shape by substrate rigidity. Cell Mol Bioeng. 2013;6:230–238. doi: 10.1007/s12195-013-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23:2434–2439. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14:824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Meek KM, Chapman JA, Hardcastle RA. Staining pattern of collagen fibrils - improved correlation with sequence data. J Biol Chem. 1979;254:710–714. [PubMed] [Google Scholar]
- Miyaishi O, Kozaki K, Iida K, Isobe K, Hashizume Y, Saga S. Elevated expression of PDI family proteins during differentiation of mouse F9 teratocarcinoma cells. J Cell Biochem. 1998;68:436–445. doi: 10.1002/(sici)1097-4644(19980315)68:4<436::aid-jcb4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, Kiessling LL. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci USA. 2014;111:13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. 2015;21:946–954. doi: 10.1038/nm.3878. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, signaling: tissue architecture regulates development, homeostasis, cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem. 1950;186:549–556. [PubMed] [Google Scholar]
- Okumura K, Hosoe Y, Nakajima N. c-Jun and Sp1 family are critical for retinoic acid induction of the lamin A/C retinoic acid-responsive element. Biochem Biophys Res Commun. 2004;320:487–492. doi: 10.1016/j.bbrc.2004.05.191. [DOI] [PubMed] [Google Scholar]
- Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, et al. Cardiovascular pathology in hutchinson-gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–U2636. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara S, Franze K, McClain CR, Wylde GW, Fisher CL, Franklin RJM, Kabla AJ, Keyser UF, Chalut KJ. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat Mater 13, 638–644. 2014 doi: 10.1038/nmat3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Raab M, Swift J, Dingal P, Shah P, Shin JW, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. 2012;199:669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeldt F, Brown AEX, Raab M, Cai SS, Zajac AL, Zemel A, Discher DE. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol. 2012;4:422–430. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- Roosa SMM, Liu YL, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26:100–112. doi: 10.1002/jbmr.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg A, Joussen S, Moser K, Hampe N, Hersch N, Hemeda H, Schnitker J, Denecke B, Lin Q, Pallua N, et al. Matrix elasticity, replicative senescence and DNA methylation patterns of mesenchymal stem cells. Biomaterials. 2014;35:6351–6358. doi: 10.1016/j.biomaterials.2014.04.079. [DOI] [PubMed] [Google Scholar]
- Shimono K, Tung WE, Macolino C, Chi AHT, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2012;18:1592. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman BW. Using kernel density estimates to investigate multimodality. J R Stat Soc Ser A. 1981;43:97–99. [Google Scholar]
- Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontology. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton JR, Doss BL, Lindsay S, Ros R. Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Sci Rep. 2016;6, 19686 doi: 10.1038/srep19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Owen TA. Relationship of cell-growth to the regulation of tissue-specific gene-expression during osteoblast differentiation. FASEB J. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- Stolz M, Gottardi R, Raiteri R, Miot S, Martin I, Imer R, Staufer U, Raducanu A, Duggelin M, Baschong W, et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat Nanotechnol. 2009;4:186–192. doi: 10.1038/nnano.2008.410. [DOI] [PubMed] [Google Scholar]
- Swift J, Harada T, Buxboim A, Shin J-W, Tang H-Y, Speicher DW, Discher DE. Label-free mass spectrometry exploits dozens of detected peptides to quantify lamins in wildtype and knockdown cells. Nucleus. 2013a;4:450–459. doi: 10.4161/nucl.27413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013b;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima K, Takaishi H, Takito J, Tohmonda T, Yoda M, Ota N, Kosaki N, Matsumoto M, Ikegami H, Nakamura T, et al. Inhibition of STAT1 accelerates bone fracture healing. J Orthop Res. 2010;28:937–941. doi: 10.1002/jor.21086. [DOI] [PubMed] [Google Scholar]
- Talwar S, Jain N, Shivashankar GV. The regulation of gene expression during onset of differentiation by nuclear mechanical heterogeneity. Biomaterials. 2014;35:2411–2419. doi: 10.1016/j.biomaterials.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Talwar S, Kumar A, Rao M, Menon GI, Shivashankar GV. Correlated spatio-temporal fluctuations in chromatin compaction states characterize stem cells. Biophys J. 2013;104:553–564. doi: 10.1016/j.bpj.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen JX, Lee CSD, Nizkorodov A, Riemenschneider K, Martin D, Hyzy S, Schwartz Z, Boyan BD. Disruption of Pdia3 gene results in bone abnormality and affects 1 alpha,25-dihydroxy-vitamin D-3-induced rapid activation of PKC. J Steroid Biochem Mol Biol. 2010;121:257–260. doi: 10.1016/j.jsbmb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- Williams JA, Kondo N, Okabe T, Takeshita N, Pilchak DM, Koyama E, Ochiai T, Jensen D, Chu ML, Kane MA, et al. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev Biol. 2009;328:315–327. doi: 10.1016/j.ydbio.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta 1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao LP, Naganawa T, Obugunde E, Gronowicz G, Ornitz DM, Coffin JD, Hurley MM. Stat1 controls postnatal bone formation by regulating fibroblast growth factor signaling in osteoblasts. J Biol Chem. 2004;279:27743–27752. doi: 10.1074/jbc.M314323200. [DOI] [PubMed] [Google Scholar]
- Yener B, Acar E, Aguis P, Bennett K, Vandenberg SL, Plopper GE. Multiway modeling and analysis in stem cell systems biology. BMC Syst Biol. 2008;2, 63 doi: 10.1186/1752-0509-2-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007;53:219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JQ, Lian QZ, Zhu GL, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang YL, Tse HF, Stewart CL, Colman A. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.