Abstract
Introduction
Most successfully resuscitated cardiac arrest patients do not survive to hospital discharge. Many have withdrawal of life sustaining therapy (WLST) as a result of the perception of poor neurologic prognosis. The characteristics of these patients and differences in their post-arrest care are largely unknown.
Methods
Utilizing the Penn Alliance for Therapeutic Hypothermia Registry, we identified a cohort of 1311 post-arrest patients from 26 hospitals from 2010 to 2014 who remained comatose after return of spontaneous circulation. We stratified patients by whether they had WLST post-arrest and analyzed demographic, arrest, and post-arrest variables.
Results
In our cohort, 565 (43%) patients had WLST. In multivariate regression, patients who had WLST were less likely to go to the cardiac catheterization lab (OR 0.40; 95% CI: 0.26–0.62) and had shorter hospital stays (OR 0.93; 95% CI: 0.91–0.95). When multivariate regression was limited to patient demographics and arrest characteristics, patients with WLST were older (OR 1.18; 95% CI: 1.07–1.31 by decade), had a longer arrest duration (OR 1.14; 95% CI: 1.05–1.25 per 10 min), more likely to be female (OR: 1.41; 95% CI: 1.01–1.96), and less likely to have a witnessed arrest (OR 0.65; 95% CI: 0.42–0.98).
Conclusion
Patients with WLST differ in terms of demographic, arrest, and post-arrest characteristics and treatments from those who did not have WLST. Failure to account for this variability could affect both clinical practice and the interpretation of research.
Keywords: Cardiopulmonary resuscitation, Heart arrest, Brain, Epidemiology, Prognosis
Introduction
Individuals who suffer cardiac arrest experience high rates of morbidity and mortality. Even when patients survive the initial arrest event, prognosis can be poor.1,2 Until the last few decades, it was assumed that the chances of regaining meaningful functional neurologic recovery in survivors who remained comatose post-arrest were low. However, with the use of more aggressive bundles of care focusing on targeted temperature management (TTM) and hemodynamic optimization, outcomes are improving, and more patients are discharged from the hospital with meaningful neurologic recovery.3–5
Despite these advances, most post-cardiac arrest patients suffer some degree of anoxic brain injury.6 This brain injury, or at least the expectation of it, is a common cause of death for post-arrest patients.7,8 The majority of successfully resuscitated post-arrest patients who remain comatose die after withdrawal of life-sustaining therapies (WLST) based on a presumed poor neurologic outcome.7,9–11 This is appropriate for patients with non-recoverable neurologic injuries, but post-arrest prognosis is difficult and it often takes many days post-arrest to determine outcomes.6,8 Indeed, remaining comatose post-arrest may lead patients to have WLST earlier than recommended for an “adequate” neuroprognostic decision to be made.9,10
Although guidelines address the need for neuroprognostication in WLST decision-making, the specific factors potentially influencing the decision to pursue WLST remain incompletely explored. To address this gap in knowledge, we sought to characterize the demographic, arrest, and post-arrest factors associated with WLST in post-arrest patients.
Methods
This is a retrospective cohort study utilizing data from the Penn Alliance for Therapeutic Hypothermia (PATH) Registry. The PATH registry is a national, online repository for patient data from multiple centers utilizing TTM in the management of post-cardiac arrest patients. This was a multi-center study evaluating patient data from 27 institutions and was approved by the University of Pennsylvania Institutional Review Board with a waiver of informed consent.
We identified adult comatose post-arrest patients between 2010–2014 from the PATH registry with information on do not resuscitate (DNR) orders and WLST. Patients were excluded if they were not successfully resuscitated post-cardiac arrest, if they were younger than 18 years of age, and if they had missing information on DNR status or outcome at hospital discharge. Patient demographic data including age, race and sex were compiled. The following patient comorbidities were abstracted: acute stroke or transient ischemic attack, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), dementia, diabetes mellitus (DM), end stage renal disease (ESRD), hypertension, metastatic or hema-tologic cancer, peripheral vascular disease (PVD), HIV/AIDS, and congestive heart failure (CHF). Finally, arrest variables (location of arrest, suspected etiology, initial pulseless rhythm, and duration of arrest), and post-arrest variables (whether the patient received TTM, had documented neurology or cardiology consultations, went to the cardiac catheterization laboratory, went to the electrophys-iology laboratory, had electroencephalography (EEG) performed, had a computerized tomography (CT) scan of the head or brain Magnetic Resonance Imaging (MRI), had echocardiography performed, and the length of hospital stay) were collected for each patient. The primary outcome, WLST, was documented in the chart by the attending critical care physician and was defined not simply as change in code status, but as the decision to actively withdraw supportive therapies and provide comfort measures only.
Differences in categorical variables by primary outcome (WLST versus no WLST) were analyzed using Chi-square tests. Continuous variables were checked for normality using the skewedness and kurtosis test for normality and then analyzed using a Mann–Whitney U test to compare the differences in medians by group. To analyze the relationship between patient- and arrest-level variables and WLST, a multivariate logistic regression model was fit using demographic and arrest factors in order to assess how these variables contribute to WLST. Covariates were included in this model if they had a p-value ≤ 0.2512,13 and removed from the model using backward elimination using Stata 12.1 (College Station, TX). Potential effect modifiers were examined and model fit was examined both with and without the interaction term(s). In order to evaluate the relationship between post-arrest care modalities and WLST while controlling for patient-level variability, a series of logistic regressions were fit controlling for the relevant demographic and arrest characteristics, as determined by the previous analysis. Tests for trend across ordered groups was performed to assess changes in rates of WLST by year and changes in percentage of WLST performed prior to 72 h post-arrest by year. As this was a multi-center study, post-estimation likelihood ratio tests were performed to evaluate the extent of clustering by site.
Results
Of 1311 patients meeting inclusion criteria, 565 (43%) patients had WLST. These patients differed in demographic, arrest, and post-arrest characteristics and treatments (Table 1). Patients with WLST were more likely to be older, female, have an unwitnessed arrest, have an initial non-shockable rhythm, and have longer duration of arrest. They were more likely to have an EEG performed and to have a shorter hospital length of stay. They were less likely to have TTM performed, a consultation from the cardiology service, go to the cardiac catheterization lab, have an MRI of the brain, or have echocardiography performed. In terms of comorbidities, patients with WLST were statistically more likely to have COPD, CAD, DM, hypertension, PVD, metastatic cancer, and CHF, but statistically less likely to have a history of acute stroke or transient ischemia attack. The median length of stay was significantly longer in the patients without WLST (WLST: 2 [IQR: 1, 5] days; no-WLST: 10 [IQR: 2, 17]; Fig. 1).
Table 1.
Comparison of patients with WLST and those who did not have WLST.
| WLST (n = 565) | No WLST (n = 746) | p-value | |
|---|---|---|---|
| Age (median [IQR] years) | 67 (56, 78) | 62 (51, 72) | <0.001 |
| Race | |||
| White | 72.7% | 67.0% | |
| Black | 21.9% | 25.0% | 0.082 |
| Other | 5.4% | 8.0% | |
| Male | 53.7% | 63.1% | 0.001 |
| Comorbidities | |||
| Acute stroke/transient ischemic attack | 2.1% | 4.3% | 0.033 |
| Chronic pulmonary obstructive disorder | 11.0% | 5.6% | <0.001 |
| Coronary artery disease | 18.8% | 14.2% | 0.026 |
| Dementia | 3.0% | 1.8% | 0.341 |
| Diabetes mellitus | 23.1% | 16.2% | 0.002 |
| Hypertension | 33.7% | 27.2% | 0.012 |
| Cancer | 6.6% | 3.9% | 0.030 |
| Peripheral vascular disease | 6.0% | 2.9% | 0.006 |
| End stage renal disease | 5.7% | 5.2% | 0.682 |
| HIV/AIDS | 0.2% | 0.0% | 0.265 |
| Congestive heart failure | 21.1% | 12.3% | <0.001 |
| Witnessed | 76.4% | 83.8% | 0.002 |
| Cardiac etiology of arrest | 61.8% | 72.3% | <0.001 |
| Out-of-hospital arrest | 58.6% | 62.7% | 0.132 |
| Initial rhythm | |||
| VF/VT | 24.8% | 43.6% | |
| Asystole | 30.3% | 20.3% | <0.001 |
| PEA | 44.9% | 36.0% | |
| Duration of arrest (median [IQR] minutes) | 20 (9, 34) | 13 (8, 25) | <0.001 |
| Targeted temperature management | 54.3% | 65.2% | <0.001 |
| Neurology consultation | 64.2% | 60.2% | 0.328 |
| Cardiology consultation | 70.9% | 81.0% | 0.005 |
| Cardiac catheterization lab | 19.5% | 43.9% | <0.001 |
| Electrophysiology lab | 1.5% | 8.6% | <0.001 |
| Electroencephalography | 52.2% | 47.8% | 0.301 |
| Head CT scan | 58.7% | 62.5% | 0.383 |
| Brain MRI scan | 4.6% | 9.5% | 0.037 |
| Echocardiography performed | 60.2% | 72.2% | 0.003 |
| Hospital length of stay (median [IQR] days) | 2 (1, 5) | 10 (2, 17) | <0.001 |
Fig. 1.
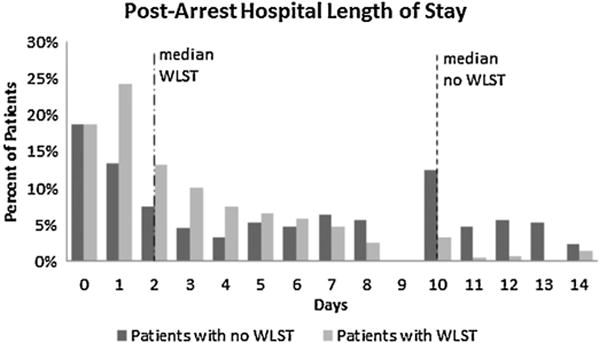
Post-arrest hospital length of stay by withdrawal of life-sustaining therapies (WLST).
When multivariate regression was limited to patient demographics and arrest characteristics (age, race, sex, whether the arrest was witnessed, duration of arrest, an interaction between etiology of arrest and initial rhythm), patients with WLST were older (OR 1.18; 95% CI: 1.07–1.31 by decade), had a longer duration of arrest (OR 1.14; 95% CI: 1.05–1.25 for each additional 10 min of pulselessness), were more likely to be female (OR: 1.41; 95% CI: 1.01–1.96), and were less likely to have a witnessed arrest (OR 0.65; 95% CI: 0.42–0.98). In multivariate regression analysis controlling for the same demographic and arrest characteristics as well as the year of arrest, patients who had WLST were less likely to go to the cardiac catheterization lab (OR 0.40; 95% CI: 0.26–0.62), and had shorter hospital stays (OR 0.93; 95% CI: 0.91–0.95 by day).
Information on timing of WLST was available in 553/565 (97.9%) patients with WLST. 294/553 (53.2%) of patients who had WLST had it occur in the first 48 h post-arrest (“early WLST”; Table 2). Patients with early WLST were more likely to be older, white, female, and have an initial non-shockable rhythm, a non-cardiac etiology of arrest, an in-hospital cardiac arrest, and a longer duration of arrest. They were less likely to have an electroencephalogram (EEG) performed, receive targeted temperature management (TTM) or a consultation from the cardiology or neurology service, go to the cardiac catheterization lab, have a head CT, or have echocardiogra-phy performed. In terms of comorbidities, patients with early WLST only differed from those without in that they were statistically less likely to have a history of acute stroke or transient ischemia attack (TIA). The median length of stay was significantly shorter in the patients with early WLST (1 [IQR: 0, 1] day vs. 8 [IQR: 3, 16] days).
Table 2.
Comparison of patients with WLST in the first 48 h post-arrest and those without WLST/WLST after 48 h post-arrest.
| WLST in 1st 48 h (n = 294) | No WLST or WLST after 48 h (n = 1005) | p-Value | |
|---|---|---|---|
| Age (median [IQR] years) | 71 (59, 80) | 62 (52, 73) | <0.001 |
| Race | |||
| White | 75.9% | 67.5% | |
| Black | 18.9% | 25.2% | 0.040 |
| Other | 5.2% | 7.2% | |
| Male | 50.9% | 61.2% | 0.001 |
| Comorbidities | |||
| Acute stroke/transient ischemic attack | 0.7% | 4.2% | 0.004 |
| Chronic pulmonary obstructive disorder | 7.8% | 8.1% | 0.902 |
| Coronary artery disease | 14.6% | 16.7% | 0.395 |
| Dementia | 2.4% | 1.8% | 0.474 |
| Diabetes mellitus | 18.7% | 19.2% | 0.843 |
| Hypertension | 28.2% | 30.2% | 0.519 |
| Cancer | 7.1% | 4.5% | 0.071 |
| Peripheral vascular disease | 5.1% | 4.1% | 0.449 |
| End stage renal disease | 3.4% | 6.1% | 0.079 |
| HIV/AIDS | 0.3% | 0.0% | 0.235 |
| Congestive heart failure | 17.7% | 15.8% | 0.438 |
| Witnessed | 81.2% | 80.5% | 0.804 |
| Cardiac etiology of arrest | 61.1% | 67.8% | 0.040 |
| Out-of-hospital arrest | 48.0% | 64.2% | <0.001 |
| Initial rhythm | |||
| VF/VT | 18.8% | 40.2% | |
| Asystole | 32.8% | 22.3% | <0.001 |
| PEA | 48.5% | 37.5% | |
| Duration of arrest (median [IQR] minutes) | 19 (8, 34) | 15 (8, 29) | 0.048 |
| Targeted temperature management | 34.4% | 68.0% | <0.001 |
| Neurology consultation | 41.3% | 67.9% | <0.001 |
| Cardiology consultation | 55.8% | 80.9% | <0.001 |
| Cardiac catheterization lab | 14.0% | 38.1% | <0.001 |
| Electrophysiology lab | 0.4% | 6.9% | <0.001 |
| Electroencephalography | 25.7% | 56.1% | <0.001 |
| Head CT scan | 37.1% | 66.4% | <0.001 |
| Brain MRI scan | 3.2% | 8.0% | 0.104 |
| Echocardiography performed | 38.0% | 73.5% | <0.001 |
| Hospital length of stay (median [IQR] days) | 1 (0, 1) | 8 (3, 16) | <0.001 |
When multivariate regression was limited to patient demographics and arrest characteristics (age, race, sex, whether the arrest was witnessed, duration of arrest, etiology of arrest, and initial rhythm), patients with early WLST were older (OR 1.34; 95% CI: 1.19–1.50 by decade), had a longer duration of arrest (OR 1.12; 95% CI: 1.03–1.21 for each additional 10 min of pulselessness), were more likely to be female (OR: 1.54; 95% CI: 1.08–2.21), were less likely to have a shockable initial rhythm (OR: 0.43; 95% CI: 0.28–0.66) and were less likely to be African–American (OR 0.56; 95% CI: 0.36–0.87). In multivariate regression analysis controlling for the same demographic and arrest characteristics as well as the year of arrest and for clustering by hospital, patients who had early WLST were less likely to be treated with targeted temperature management (OR: 0.37; 95% CI: 0.21–0.63) to have a neurology consultation (OR: 0.31; 95% CI: 0.20–0.47), to have a cardiology consultation (OR: 0.35; 95% CI: 0.20–0.62), go to the cardiac catheterization lab (OR 0.29; 95% CI: 0.17–0.50), have a head CT (OR: 0.27; 95% CI: 0.15–0.50), have echocardiography performed (OR: 0.20; 95% CI: 0.13–0.30), and have EEG performed (OR: 0.24; 95% CI: 0.13–0.45). We compared demographic and arrest characteristics in patients by timing of WLST/death in non-survivors (first 2 days, days 3–7, and after day7) and in survivors and found no clear systematic differences or trends (Supplemental Table S1 in the online version at DOI:http://dx.doi.org/10.1016/j.resuscitation.2016.10.021). We also limited the population to OHCAs and found little change in associations in most univariate and multivariate analyses (Supplemental Tables S2 and S3 in the online version at DOI:http://dx.doi.org/10.1016/j.resuscitation. 2016.10.021). Of note, the associations between race and sex in the early WLST group were no longer significant, and OHCA patients with early WLST were found to receive significantly fewer brain MRIs.
There was a significant trend toward increased rates of WLST by year (Fig. 2). However, there was no significant difference in the rates of WLST prior to 72 h post-arrest by year. Our statistical evaluation of clustering by site did not yield evidence of any changes in association.
Fig. 2.

Percentage of patients with withdrawal of life-sustaining therapies by year.
Discussion
Our findings show that, in adjusted analysis, patients with withdrawal of life-sustaining therapies were older and more likely to be female, have a longer duration of arrest, and to have had an unwitnessed arrest. They were also more likely to have certain comorbidities: COPD, CAD, DM, hypertension, PVD, metastatic cancer, and CHF. This analysis clearly outlines that patients with WLST have different characteristics than those that do not. Additionally, in patients with WLST, those with “early WLST” were older and more likely to be female, have a longer duration of arrest, have an initial non-shockable rhythm, and less likely to be African–American than those with later WLST. They were also less likely to have experienced an acute stroke or TIA. Whether this is the effect of provider bias or the result of pathophysiologic difference cannot be inferred from this study; however, our analysis accounted for physiologic difference between cohorts.
Our results are similar to a recent publication looking at early WLST versus late WLST post-arrest. This study found that 52% of comatose post-arrest patients had WLST and that the decision to withdraw these therapies was influenced by age, race, preexisting comorbidities, multi-organ failure, and a poor initial neurologic exam.14 Although we did not investigate the effect of multi-organ failure or the initial neurologic exam, we did find similar results for age, with older patients more likely to have WLST and more likely to have early WLST. We did not find a statistically signifi-cant relationship between race and WLST, but we did find that a larger proportion of patients who had WLST versus no WLST were white (72.7% vs. 67.0%), a trend that was reversed in African Amer-ican patients (21.9% vs. 25.0%) (p = 0.08). Although not statistically significant, this trend does allude to a possible racial disparity in the utilization of WLST. Additionally, African Americans were significantly less likely to have WLST in the first 48 h. In contrast to the above work, which found no difference between patients with certain cormorbidities,14 we found that patients with certain comorbidities (specifically, those with COPD, CAD, DM, hypertension, PVD, metastatic cancer, and CHF) were more likely to have WLST, a difference that could be explained by comparative sample sizes.
Looking at the utilization of withdrawal of life-sustaining therapy is of vital importance in post-arrest care—it is not only prevalent,11 but also variable. A study done by Sandroni et al. that explored the application of neuroprognostic tools in patients treated with therapeutic hypothermia found that the quality of evidence supporting the use of these tools ranged from “Very Low” to “Moderate”, and that none were good predictors of neurologic recovery. The authors concluded that in the first 7 days post-arrest, some of these tools, such as a bilaterally absent N20 somatosensory evoked potential (SSEP) wave or a nonreactive EEG after rewarming, were useful for predicting poor neurologic outcome, but that these tools were accompanied by a high risk of bias.3 Unfortunately, many patients have WLST prior to 7 days post-arrest – median time to WLST was only 2 (IQR: 1, 5) days post-arrest in this study and was previously documented as 3 (IQR:1–5) days in another13 – which results in decisions prior to the application of these prognostic tools and the possibility of death in a patient who may have had a different outcome if given further time to awaken or undergo further neuroprognostic testing.
Despite the imprecision of post-arrest neuroprognostic tools, many patients who survive initial resuscitation die as a result of WLST due to suspected neurologic causes. One study, which looked at the cause of death in ICU-admitted post-arrest patients who died before hospital discharge, found that suspected neurologic injury was the cause of death in 58/126 non-survivors (46%), which differed based on location of arrest: 68% of patients with out of hospital arrests had suspected neurologic injury as the cause of death compared to 23% of patients with in-hospital arrests.7 A similar study looked at 58 patients and found that 40 patients died as a result of withdrawal of life-sustaining therapies, 8 died as a result of brain/cardiac death, and 10 survived, which means that 83% of non-survivors had WLST—showing how common this practice is. Another study of 55 TTM-treated patients with arrests between 2005–2009 found that 57% patients had a negative neurologic prognosis within 15 h after being rewarmed; 25% of these had WLST prior to 72 h post-arrest. Most astonishingly, 21% of the patients given a poor prognosis had a good neurologic outcome at hospital discharge9; showing how important understanding the mechanisms behind WLST is both to patients and to clinical research.
As medical resources are finite, it is important to recognize that some post-arrest patients will not have a reasonable hope of recovery and may not benefit from the continued use of considerable resources that could be allocated elsewhere. However, there is a lack of standardized protocol for determining these patients and deciding to withdraw life sustaining therapy within and between institutions as well as a lack of consensus for what this protocol should entail. In a large randomized controlled trial (RCT) in Europe examining two different target temperatures for post-arrest targeted temperature management,3 Nielsen et al. actively worked to reduce the potential bias by using a protocol across the entire RCT. This protocol required that all patients be actively treated until 72 h after the intervention period (108 h post-arrest) and then specified when neurological evaluation would be done on comatose patients, protocolized what the examination would entail, and documented the rationale for all WLST,11 showing that it is possible to adopt a well-defined standard protocol for WLST. Additionally, no patient could have therapy withdrawn for neurologic reasons prior to 72 h post-arrest, except in cases of cerebral herniation or early myoclonus status with a negative SSEP.3 This protocol differs vastly from what we found in this study—contrary to waiting 4.5 days prior to beginning to assess patients for WLST as stated in the protocol, patients had life-sustaining therapies withdrawn a median of 2 days post-arrest, which is consistent with findings in in-hospital patients.15 This disconnect and the variability in prac-tice highlights the importance of accounting for WLST and delving further into the mechanisms behind current practices.
However, a recent multi-center randomized controlled trial aimed at improving adherence to neuroprognostication protocols showed that a quality improvement intervention increased rates of appropriate neuroprognostication. Although this trial did not show significant improvement in survival, it does provide support for the feasibility of successful implementation of a standardized protocol.16
Without standardization, the results of cardiac arrest research, especially those with neurologic status as a primary or secondary outcome, could be severely biased. We have shown that there is variability of care around WLST, which can lead to self-fulfilling prophecies in which life-sustaining therapies are withdrawn in patients with the potential to recover neurologically. This can lead to an overestimation of the ability of a test to predict bad outcome and affect the modalities used to neuroprognosticate. Neuroprog-nostic tests are also usually ordered for a specific reason, which could lead to spectrum bias, causing an over- or under-estimation of the utility of the prognostic tools being applied when making the decision to WLST. A recent study of 16,875 OHCAs estimated that early withdrawal of care due to expected poor neurologic prognosis was associated with an annual excess mortality of 2300 patients in the US, 64% of whom may have had a favorable functional outcome (as measured by a modified Rankin score 3 at hospital discharge).17 Not accounting for the effects of WLST ≤ variability is potentially harmful both clinically and in resuscitation research, especially given our finding that there is a trend over time toward increased WLST.
There were multiple limitations in our study; most notably, this investigation was an analysis of retrospectively collected data. Relying on medical records and documentation with the purpose of patient care as opposed to research inevitably leads to miss ing data and the potential for misclassification. Additionally, the use of registry data limits data to only pre-specified and defined data points and can lead to a loss of nuance by limiting response choices. As there is currently no standard practice for WLST and this is a multi-center study, protocols, practice, and patient composition could vary widely by site and add spurious heterogeneity, although we did not find significant evidence of this. However, use of a registry allowed for this evaluation of over 1300 patients at 26 American institutions, the largest study of WLST in this population. Finally, without thorough documentation of the processes that went into the decision to withdraw life-sustaining therapies, particularly because our data set does not delineate which patients had WLST due to neurologic poor prognosis versus medical futility, we have no way to determine which factors were deemed important by the healthcare proxy in ultimately deciding whether to withdrawal life-sustaining therapies in a particular patient.
Conclusions
In conclusion, comatose post-arrest patients who had WLST in the hospital were older, were more likely to have a longer arrest downtime, be female, have an unwitnessed arrest, and have COPD, CAD, DM, hypertension, PVD, metastatic cancer, and CHF. They are more likely to have post-arrest neurology and cardiology consults, less likely to go to the electrophysiology lab, and have a shorter hospital stay. Further investigation is necessary to understand the intricacies that contribute to decisions surrounding WLST as well as the timing of decision in post-arrest patients who remain comatose despite post-cardiac arrest care.
Supplementary Material
Acknowledgments
We would like to thank the members of the Penn Alliance for Therapeutic Hypothermia (PATH) registry who contributed cardiac arrest data from their institutions.
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2016.10.021.
Conflict of interest statement
Dr. Grossestreuer receives research support from the Ameri-can Heart Association. Dr Gaieski provides consulting for CR Bard and Stryker Medical and has received honoraria from CR Bard. Dr. Abella has received research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute, American Heart Association and CR Bard, in-kind research support from Laerdal Medical Corporation, and honoraria from CR Bard. Dr. Moskowitz receives research support from the National Institutes of Health (#T32HL007374-37). Dr. Haukoos receives research support from the National Institutes of Health. Dr. Perman receives research support from the National Institutes of Health. Dr. Wiebe and Mr. Ikeda declare no conflict of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–92. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–9. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 ◦C versus 36 ◦C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 4.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Peri-operative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–79. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Callaway CW, Schmicker RH, Brown SP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85:657–63. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: patients treated with therapeutic hypothermia. Resuscitation. 2013;84:1324–38. doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–8. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 8.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337–42. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Perman SM, Kirkpatrick JN, Reitsma AM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit Care Med. 2012;40:719–24. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–8. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 11.Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42:2493–9. doi: 10.1097/CCM.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 13.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907–16. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 14.Albaeni A, Chandra-Strobos N, Vaidya D, Shaker ME. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85:1455–61. doi: 10.1016/j.resuscitation.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 16.Scales DC, Golan E, Pinto R, et al. Improving appropriate neurological prognostication after cardiac arrest: a stepped wedge cluster RCT. Am J Respir Crit Care Med. 2016 Apr; doi: 10.1164/rccm.201602-0397OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–35. doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


