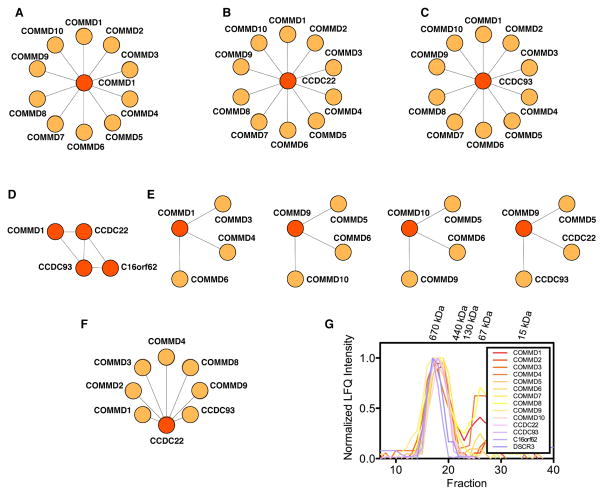
Figure 3.
Evidence for Commander from small-scale proteomic studies. (A)-(F) Examples of published bait (red) and prey (orange) experiments with Commander subunits. (A) COMMD1 interacts with itself and all other COMMD proteins (Burstein et al., 2005). (B) CCDC22 binds COMMD1-10 (Starokadomskyy et al., 2013). (C) CCDC93 interacts with COMMD1-10 (Phillips-Krawczak et al., 2015). (D) A CCC complex of COMMD1-CCDC22-CCDC93 bound to C16orf62 has been extensively biochemically characterized, and is involved in endosomal protein trafficking (Bartuzi et al., 2016; Li et al., 2015; Phillips-Krawczak et al., 2015). (E) Other combinations of COMMDs have been seen to preferentially interact, for example COMMD3, -4 and -6 were identified in an early COMMD1 tandem affinity purification (TAP) screen (Burstein et al., 2005; Starokadomskyy et al., 2013). (F) We have verified several of the novel protein-protein interactions in the Commander complex by AP-MS (Wan et al., 2015). (G) Size exclusion chromatography of Commander measured by MS (Kirkwood et al., 2013). Elution profiles of all 14 putative Commander subunits indicate that they co-elute as a single entity of approximately 600 kDa in mass.