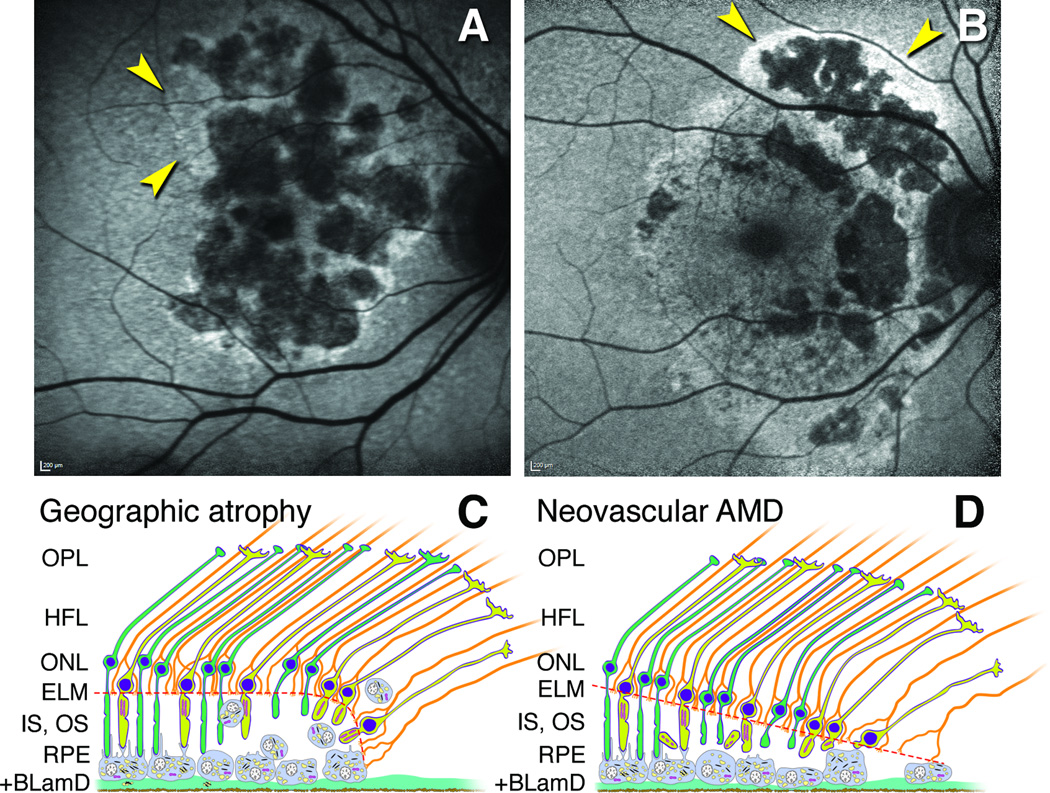
Figure 7. Proposed mechanisms for hyperautofluorescence in primary geographic atrophy and macular atrophy secondary to neovascularization in age-related macular degeneration.
A. Multilobular GA with variegated hyperautofluorescence in the margins (arrows). B. Neovascular AMD with multilobular atrophy and also hyperautofluorescence in a floodplain configuration (arrows), attributed to the history of exudation in this eye (image courtesy of N. Phasukkijwatana, MD, and D. Sarraf, MD). C,D. Schematics show outer retinal cells at the descent of the ELM (orange dashed line) towards Bruch’s membrane in GA (C) and nvAMD (D). The ELM is comprised of junctional complexes of Müller cells (orange) and photoreceptors (yellow, cones; green, rods). For simplicity, Bruch’s membrane and neovascular membranes are not shown. C. The RPE layer exhibits a progressive dysmorphia towards the ELM descent.27, 30, 31, 54 Hyperautofluorescence is attributable to stacked and rounded RPE cells that increase path length of exciting light through biologic fluorophores.31 D. In nvAMD, the RPE is non-uniform in morphology, without worsening toward the border. Photoreceptor loss is severe due to inner segment shedding40 believed secondary to episodic exudation. Reduced screening by photopigment in these shortened cells also contributes to hyperautofluorescence. In most cases, as in panel B, RPE dysmorphia and reduced photopigment screening may occur simultaneously.