Figure 1.
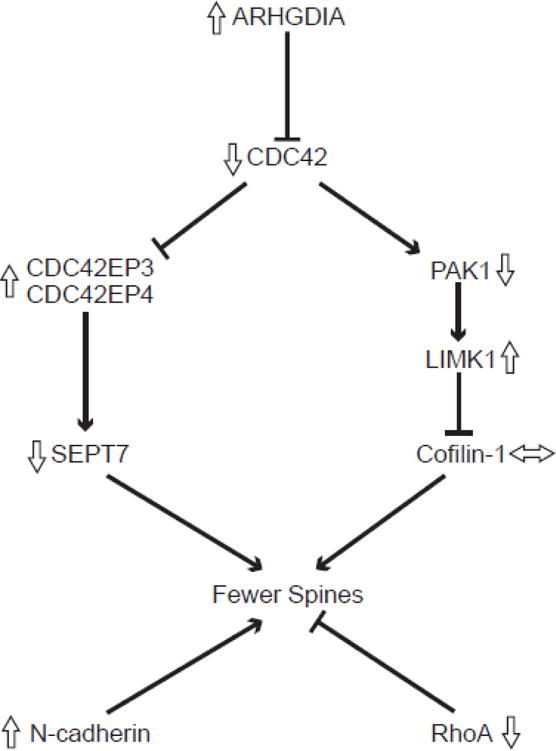
Signaling pathways for selected transcripts that regulate dendritic spine density. Lines with arrowheads indicate activation and those with blunted ends indicate inhibition. Open arrows indicate the direction of altered expression of each transcript in layer 3 pyramidal cells in schizophrenia (Arion et al., 2015; Datta et al., 2015a; Ide and Lewis, 2010). The protein products of these transcripts contribute to spine stability through regulation of the assembly and stability of actin filaments (Koleske, 2013). Rho GTPases are dendritically translated, are active in the spine, and have well elucidated roles in the morphogenesis and maintenance of dendritic spines, including molecular signaling cascades such as the cell division cycle 42 (CDC42) pathway (Tada and Sheng, 2006). In this pathway, CDC42 is negatively regulated by a Rho GDP dissociation inhibitor alpha (ARHGDIA) (Gorvel et al., 1998) and acts through the CDC42-p21-activated serine/threonine kinase (PAK)-LIM domain kinase (LIMK) pathway to promote spine stability by phosphorylating and deactivating the neuronal cofilin, cofilin-1, an actin severing protein (Bernstein and Bamburg, 2010). In a separate pathway, negative regulation by CDC42 on CDC42 effector proteins 3 and 4 (CDC42EP3 and CDC42EP4) promotes spine outgrowth by opening the barrier at the base of dendritic spine necks formed by septin molecules such as Septin-7 (SEPT7) and allowing influx of molecules important in spine growth into the spine head (Joberty et al., 2001). RhoA is another GTPase which has a negative influence on dendritic spine maintenance, contributing to spine loss (Tashiro et al., 2000). In addition to the role that GTPases play in dendritic spine maintenance, other molecules contribute to the stability of spines. For example, neuronal cadherin (N-cadherin) has been shown to contribute significantly to nascent synapse stabilization and subsequent spine formation (Takeichi, 2007).
