Abstract
Cobra neurotoxin, a short-chain peptide isolated from snake venom of Naja naja atra, showed both a central analgesic effect and a hyperalgesic effect in mice tests. In order to explore mechanisms, a hypothesis is put forward that cobra neurotoxin takes effect through adenosine receptor pathway. The central effects of cobra neurotoxin were evaluated using the hot plate test (a model of acute pain) and the spinal cord injury (a model of central pain) in mice and using A1 receptor antagonist (DPCPX) and A2A receptor antagonist (ZM241385); behaviors were scored and signal molecules such as reactive oxygen species and adenosine triphosphate levels and mitogen-activated protein kinases/extracellular signal-regulated protein kinase expression were measured. Low dose of cobra neurotoxin (25 µg/kg) had analgesic effects which were inhibited by DPCPX, while high dose of cobra neurotoxin (100 µg/kg) had hyperalgesic effects which were blocked by ZM241385. Cobra neurotoxin reduced reactive oxygen species and increased adenosine triphosphate in brain tissues, and extracellular signal-regulated protein kinase expression was markedly inhibited by cobra neurotoxin. Cobra neurotoxin may take effect through mitogen-activated protein kinases/extracellular signal-regulated protein kinase pathway inhibition by activating adenosine A1Rs and cause changes of reactive oxygen species and adenosine triphosphate through feedback mechanisms. Overdose cobra neurotoxin further activates the adenosine A2ARs to generate pain sensitization. This research proposes a new central analgesic mechanism of cobra neurotoxin and discloses dual regulation of pain.
Keywords: Cobra neurotoxin, central analgesia, hyperalgesia, adenosine receptors, two ways regulation
Introduction
Chronic pain, a complex condition caused by inflammation, nerve injury, and cancer, is associated with multiple signaling molecules in cells, including hormones, cytokines, neurotransmitters, lymphatic factors, growth factors, and chemical inducers which could change the activity of ion channels, relevant enzymes, and gene expression.1–3 Current analgesic therapies for pain involve several shortcomings such as gastrointestinal effects, tolerance, and dependence,4 and there is considerable interest in exploring novel drug targets. This includes herbal natural products and marine/terrestrial neurotoxins.5,6 Cobra neurotoxin (CNT), a short-chain peptide isolated from snake venom of Naja naja atra, has a central analgesic effect without producing tolerance or dependence and shows prospects for clinical application.7,8 CNT is generally regarded as an antagonist to cholinergic receptors due to its inhibition on inflammatory pain weakened by nicotinic acetylcholine receptors antagonist.9 However, recently it has been reported that CNT causes trigeminal neuralgia at higher dose,10 which is contrary to such a mechanism. CNT, like other toxin,11 may exhibit dual pain regulation, with analgesia and hyperalgesia in different physiological conditions or dosage through interaction with different receptors and signal pathways. Due to uncertain sites of action and unclear signal transduction pathway of two ways regulation, the antinociceptive effect of CNT may involve pathways and mechanisms other than cholinergic receptors and opioid receptors.
Adenosine receptors are G-protein-coupled receptors and widely distributed in brain tissues and are relevant to pain.12 Adenosine can affect pain signaling via A1, A2A, A2B, and A3 receptors. A1R agonists are antinociceptive in various preclinical pain models; A2AR agonists not only exhibit peripheral pronociceptive effects but also act on immune cells to suppress inflammation; A2BR agonists show peripheral proinflammatory effects on immune cells; A3Rs can produce antinociception with mechanistic actions on glial cells.13 A1 and A2A receptors play an important role in the pain signal transduction,14,15 and adenosine receptors can exhibit dual regulation of pain.14,15 Moreover, caffeine, a nonselective inhibitor of adenosine receptors, could reduce nicotinic acetylcholine receptors-mediated response (increase of transmembrane current and opening of ion channels) that has been thought as the analgesic mechanism of CNT.16 There may be a relationship between adenosine receptors and CNT, which intrigues us to disclose the role of adenosine receptors in the central analgesic action of CNT. Since CNT can inhibit inflammation,17 which is opposite to proinflammatory effects of A2BRs, and a majority of A3Rs has been found in peripheral tissues rather than central nerve system,18 this research focuses on its relation with A1Rs and A2ARs.
In this study, we examined the hypothesis that CNT acts through adenosine A1 and A2A receptors and their transduction pathways. The hot plate test and spinal cord injury (SCI) model were used in this study to explore differences in the upstream signal molecules such as reactive oxygen species (ROS) and adenosine triphosphate (ATP) level, and downstream signal molecules such as mitogen-activated protein kinases (MAPK)/extracellular signal-regulated protein kinase (ERK).
Materials and methods
Chemicals and reagents
The crude cobra venom was purchased from Jiangxi Snake Farm (Jiangxi, China), and CNT was isolated and purified in our previous research.19 Morphine was bought from Northeast Pharmacy Group (Shenyang, China). Pentobarbital sodium and amoxicillin sodium were bought from Guangzhou Qiyun Biotech Company (Guangzhou, China). A1 receptor antagonist (DPCPX, 98%) and A2a receptor antagonist (ZM241385, 98%) were bought from Sigma (St. Louis, MO, USA). BCA protein assay kit, ROS kit, and ATP kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Phospho-p44/42 MAPK (Erk1/2), GAPDH antibody, and horseradish peroxidase-linked antibody were bought from Cell Signal Technology Inc., Beverly, MA, USA. Other reagents were all analytical reagents and purchased from Guangzhou Feibo biotechnology company (Guangzhou, China).
Animals
The experiments were performed on Kunming mice of weight 20 ± 2 g, supplied by Experimental Animal Center of South Medical University. The animals were housed under conditions of 25℃ ± 2℃, 50% ± 10% humidity with a 12 h light/dark cycle. Food and water were accessible ad libitum. The experiments have been performed in accordance with the Chinese Guidelines for the use of laboratory animals and received approval from the Animal Experimentation Ethic Committee of South China University of Technology. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Analgesic test of CNT
Analgesic effects of CNT were evaluated by the hot plate test according to the reference.20 Because the scrotal skin of male mice is sensitive to heat, the mice are prone to jumping and the phenomenon of licking the hind paw is not easily observed, only female mice were chosen to keep the pain threshold stable in the same group. The female mice were divided into five groups of 18 mice for each: normal saline, morphine, CNT at high dose (HD), middle dose (MD), and low dose (LD), respectively. The medicated groups of mice were intraperitoneally injected with morphine 10 mg/kg, CNT at 25 µg/kg (LD), 50 µg/kg (MD), and 100 µg/kg (HD) based on LD50 of CNT (about 200 µg/kg) according to the reference.21 Half of LD50 was chosen as the HD in consideration of safety, and 1/4 LD50 and 1/8 LD50 were used as medium and LD, respectively. No irritant and toxic effects on normal mice such as dyspnea, bite, piloerection, pale skin, or lazy were observed at these doses.22 The control group was administered with the same volume of normal saline; 4 h later, each group was divided into three groups with six mice each and intraperitoneally injected with saline (0.1 mL/10g), DPCPX (2 mg/kg), or ZM241385 (1 mg/kg) separately. The latency time for paw licking or jumping was recorded as pain threshold in the hot plate test (surface temperature 55℃ ± 0.5℃). Basic pain threshold was determined before administration, and the responses were recorded at 0.5, 1, 2, 4, 4.5, 5, 6, and 8 h after administration. Pain threshold increase (%) = (Pt–P0) × 100/P0, where P0 and Pt are separately basic pain threshold and pain threshold at “t” time interval.
SCI in mice
SCI was used for a central pain model with some modifications.23 Contusion was performed at the vertebrae T10 level of mice using extradural vascular compression after intraperitoneal injection of 50 mg/kg of 1% pentobarbital sodium for anesthesia. The skin and the paravertebral muscle of vertebrae T9 to T11 were dissected. The laminectomy was carried out at the spinal T10 segment, and the spinal columns of T9 and T11 were fixed by tissue forceps. Then, one minute of extradural perstriction with a vascular clip was performed around the exposed spinal cord in order to make an acute compression injury. Subsequently, the muscle and skin were sutured in layers, and the animals were placed on a warm pad to recover from the anesthesia. The postoperative micturition was handled twice a day until spontaneous urination recovery. Amoxicillin sodium was injected intraperitoneally at 100 mg/kg once a day for the first three days to prevent a wound infection.
Three days after operation, the mice of half male and half female were randomly divided into nine groups of eight mice for each: normal control, sham group, spinal cord compression injury control group, six medicated groups including high and LDs of CNT, CNT+DPCPX, and CNT+ZM241385, respectively. Except for the absence of compression injury, the sham group was the same as the surgical procedure. Medicated groups of mice were intraperitoneally injected with CNT at 100 µg/kg·d (HD) and 25 µg/kg·d (LD) for two weeks. Normal control, sham group, and injury group were administrated with the same volume of normal saline instead. Mice in CNT+DPCPX and CNT+ZM241385 groups were intraperitoneally injected with DPCPX (2 mg/kg) and ZM241385 (1 mg/kg) 1 h later after administration of CNT. Subsequently, the Basso Mouse Scale (BMS) score of all groups was measured for two weeks. After the last administration, mice were scored and then sacrificed by decapitation. The hippocampus, corpus striatum, and spinal cord were quickly collected and conserved in −80℃ for further analysis.
Locomotive behavior evaluation
The BMS, a 9-point scale score in the process of locomotor recovery, was performed to evaluate the locomotive behavior of SCI model mice.24 No ankle movement to larger ankle movement was scored 0 to 2, the improvement of step and plantar placing occurred as score 3 to 4, and score 5 to 8 was under the circumstances of paw standing position, hindlimb-forelimb coordination, and trunk stability. Score 9 indicated the normal locomotion with trunk stability. The average score of left and right hindlimb was recorded as the BMS score of each mouse, and then the mean score of a group was calculated. The animals were scored before injury, once per day from one day to four days and then every two days till two weeks after SCI surgery.
Determination of ROS and ATP in brain tissues
ROS and ATP are important signal molecules in central pain pathway and determined by the ELISA method according to kit descriptions. The reagent of DCFH-DA (2,7-dichlorofluorescin diacetate) can be oxidized by ROS into DCFH with detected fluorescence emission. Brain tissues (1 mg) were homogenized with 20 µL of 0.1 M PBS (pH 7.4) and centrifuged at 2500 g for 10 min. After the amount of protein was determined by the BCA protein assay kit, ROS activity of brain tissue was measured with fluorescence intensity at 525 nm after excitation at 500 nm by F-4500 fluorescence spectrometer (Hitachi Company, Tokyo, Japan). ATP activity of brain tissue was determined with absorbance at 636 nm by UV-3010 ultraviolet spectrophotometer (Hitachi Company, Tokyo, Japan).
Determination of MAPK/ERK in brain tissue
Adenosine receptors have dual regulation of pain as the same as CNT’s actions, suggesting that they are both involved in pain signaling pathways. Corpus striatum as a major site of adenosine receptors is selected for determination of signal molecules to reveal the interactive effects of adenosine receptors and CNT on pain signaling pathways.25 Western blot analysis was applied to evaluate the phosphorylation level of MAPK ERK1/2 following the procedure in the reference.26 Corpus striatum of each group was homogenized in lysis buffer on an ice bath and centrifuged at 16,000 g for 5 min at 4℃. The supernatant was collected and assayed for protein content by the BCA kit. Proteins (30 µg) were electrophoresised on 5% stacking gel and 12% running gel and transferred to a 0.22 µm polyvinylidene difluoride membrane (100 V for 2 h). Membranes were blocked with 3% BSA for 2 h at room temperature. The primary antibodies were added overnight at 4℃ in a fresh blocking buffer. The membranes were washed and then covered by the horseradish peroxidase-linked antibody in tris-buffered saline with Tween for 2 h at 37℃. The membrane was washed in tris-buffered saline with Tween and detected by enhanced chemiluminescence with GADPH as the control. The optical densities of specific bands were measured with an imaging analysis system (Tanon-4200SF, Tanon Science & Technology Co., Ltd., Shanghai, China).
Statistical analysis
Data were expressed as mean ± standard deviation () and analyzed with SPSS17.0 software (IBM, Armonk, NY, USA). Significant tests among the groups were based on one-way analysis of variance and Student–Newman–Keuls test.
Results
Analgesia and hyperalgesia induced by CNT
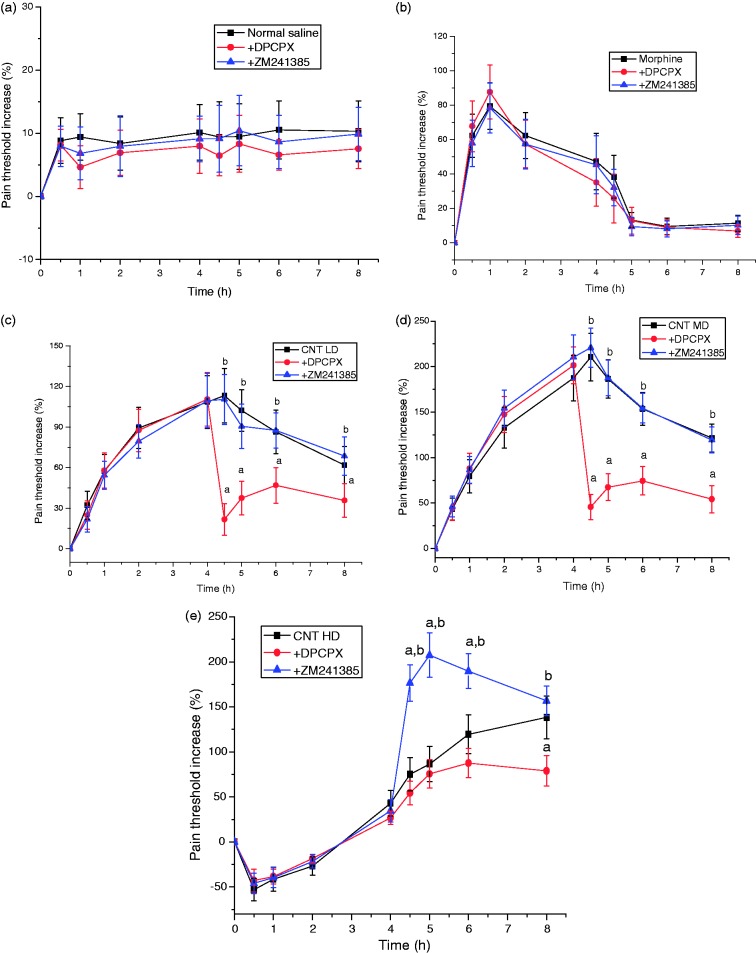
There was marked differences of pain threshold among groups treated with CNT in different doses (Figure 1). Pain threshold reached the maximum at 1 h after administration but decreased quickly in morphine group. It reached the maximum at 4.5 h after injection of CNT at LD or MD and maintained high levels at 8 h after administration. However, pain threshold was lower in the group treated with HD of CNT in the initial 3 h of administration and gradually increased thereafter. It suggests that LD and MD of CNT have long lasting and effective central analgesic effects, but HD of CNT causes hyperalgesia at earlier stage, but gradually makes analgesic effects after metabolism for a certain time.
Figure 1.
Analgesic effects of cobra neurotoxin (CNT) on mice by hot plate test. A1 receptor antagonist (DPCPX) and A2A receptor antagonist (ZM241385) were injected at 4 h after administration. (a) Normal saline, (b) morphine (10 mg/kg), (c) CNT LD (25 µg/kg), (d) CNT MD (50 µg/kg), (e) CNT HD (100 µg/kg). Data were represented as , n = 6. a, p < 0.05, compared with CNT group at corresponding dose; b, p < 0.05, compared with DPCPX group.
The adenosine A1 receptor antagonist (DPCPX) and A2A receptor antagonist (ZM241385) were given to mice after 4 h administration when the analgesic effects of CNT were still prominent. Results showed that the pain threshold in the normal group and the morphine group had no significant difference (Figure 1(a) and (b)), but obviously decreased in CNT groups after DPCPX treatment (Figure 1(c) to (e)). ZM241385 significantly increased pain threshold in CNT HD group but had no effect on CNT LD and MD groups. Results indicate that analgesic effect of CNT involves A1Rs, and the hyperalgesic effect involves A2ARs.
Influence of CNT on mice behavior
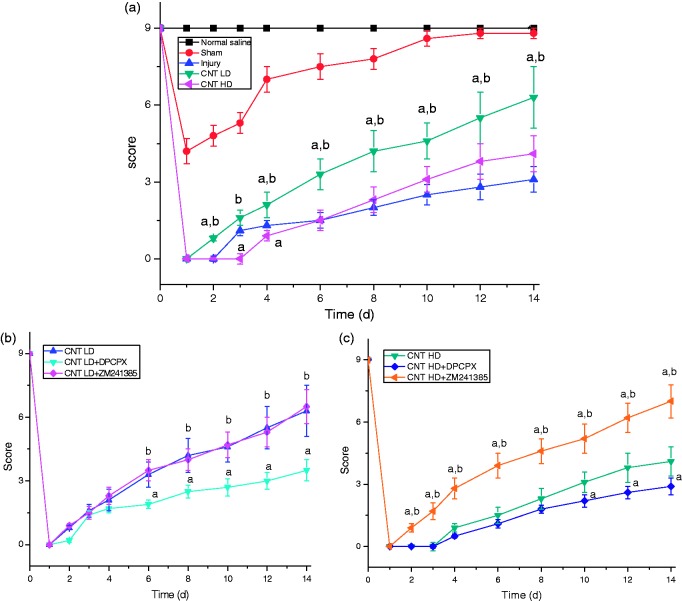
The BMS score was based on the Basso-Beattie-Bresnahan scoring system with some modifications to develop a suitable scoring method for mice behavior.24 No significant difference was found with gender on pain response, all mice for SCI model showed a maximum BMS score (score 9) during assessment before the operation (Figure 2). On the first day after surgery, all the groups with SCI showed flaccid paralysis with no hindlimb movement (BMS score 0). The sham group recovered quickly to normal level, and the compression injury animals appeared a slight ankle movement on the third day after surgery (BMS score 1) and gradually recovered up to dorsal stepping. CNT groups at LD showed extensive ankle movement (BMS score 2) on the third day and appeared frequent dorsal stepping (BMS score 3) or occasional plantar stepping (BMS score 4) after seven days administration. HD of CNT groups had lower score than the compression injury group during the earlier six days administration but exceed them thereafter. The result showed that CNT could obviously improve the locomotive performance of mice after SCI at LD, but HD led to hyperalgesia blocking motor improvement.
Figure 2.
Score of mice with spinal cord injury by Basso Mouse Scale. (a) No antagonists, (b) low dose of cobra neurotoxin (CNT LD), (c) high dose of cobra neurotoxin (CNT HD). Mice groups of CNT LD and HD received 25 µg/kg and 100 µg/kg i.p., respectively; 1 h later after administration of CNT, mice in CNT+DPCPX or CNT+ZM241385 groups were intraperitoneally injected with DPCPX (2 mg/kg) or ZM241385 (1 mg/kg). Data were represented as , n = 8. a, p < 0.05, compared with compression injury group in figure (a) and CNT at corresponding dose in figure (b) and (c); b, p < 0.05, compared with high dose group in figure (a) and DPCPX group in figure (b) and (c).
Effects of CNT on ROS and ATP level in brain tissues
ROS and ATP were measured in corpus striatum, hippocampus, and spinal cord of mice. Compared to the normal group, the level of ROS significantly increased in the compression injury group, but there was no significant difference among corpus striatum, hippocampus, and spinal cord indicating a widespread damage of mitochondria in central tissues, whereas the level of ATP decreased with obvious difference between corpus striatum and hippocampus, spinal cord suggesting that ATP breakdown to be adenosine in corpus striatum, where is the major distribution of adenosine receptors. Under pathological conditions in the nervous system, excess ROS result in the damage of mitochondria and neurons and inevitably lead to ATP degradation and more adenosine in cells. After administration of CNT, the concentration of ROS decreased markedly and the concentration of ATP improved remarkably in the corpus striatum of CNT LD group mice (Table 1). DPCPX both reduced ROS and increased ATP in the mice brain tissues of CNT LD and HD groups, but ZM241385 only significantly influenced CNT HD group. Results indicate that CNT generates a central analgesic effect through interaction with A1Rs and generates hyperalgesic effect in HD through interaction with A2ARs. The reduction of ROS and increase of ATP leading to decreased adenosine might result from interaction of CNT with A1 and A2A receptors.
Table 1.
Effects of CNT on ROS and ATP in brain tissue of mice.
| Groups | Corpus striatum |
Spinal cord |
Hippocampus |
|||
|---|---|---|---|---|---|---|
| ROS (Y/mg protein) | ATP (µmol/mg protein) | ROS (Y/mg protein) | ATP (µmol/mg protein) | ROS (Y/mg protein) | ATP (µmol/mg protein) | |
| Normal saline | 96 ± 9a | 861 ± 62a | 87 ± 8a | 376 ± 32a | 78 ± 9a | 436 ± 40a |
| Sham | 116 ± 12a | 747 ± 68a | 127 ± 14a | 361 ± 29a | 108 ± 11a | 397 ± 35a |
| Injury | 239 ± 20 | 308 ± 26 | 224 ± 21 | 69 ± 7 | 250 ± 20 | 64 ± 8 |
| CNT LD | 136 ± 16a | 660 ± 79a, | 164 ± 17a | 282 ± 13a | 162 ± 18a | 305 ± 35a |
| CNT LD+DPCPX | 222 ± 29b | 358 ± 39b | 218 ± 19b | 124 ± 16a,b | 234 ± 22b | 130 ± 16a,b |
| CNT LD+ZM241385 | 152 ± 16a | 625 ± 78a | 180 ± 19a | 259 ± 31a | 175 ± 19a | 277 ± 32a |
| CNT HD | 187 ± 17a | 465 ± 56a | 191 ± 17a | 191 ± 17a | 190 ± 17a | 226 ± 13a |
| CNT HD+DPCPX | 202 ± 19 | 321 ± 21b | 214 ± 17 | 113 ± 8a,b | 235 ± 31b | 183 ± 19a,b |
| CNT HD+ZM241385 | 134 ± 16a,b | 708 ± 72a,b | 155 ± 18a,b | 378 ± 39a,b | 153 ± 20a,b | 315 ± 30a,b |
Mice groups of CNT LD and HD received 25 µg/kg and 100 µg/kg i.p., respectively; 1 h later after administration of CNT, mice in CNT+DPCPX or CNT+ZM241385 groups were intraperitoneally injected with DPCPX (2 mg/kg) or ZM241385 (1 mg/kg). Data were represented as , n = 8. a, p < 0.05 as compared to compression injury group; b, p < 0.05 as compared to CNT group at corresponding dose.
CNT: cobra neurotoxin; LD: low dose; HD: high dose.
Influence of CNT on MAPK/ERK pathway
The phosphorylation level of ERK1 (p44 MAPK) and ERK2 (p42 MAPK) were determinate by western blotting (Figure 3); the level of p-ERK1/2 in the SCI compression injury group (lane 3) significantly increased compared to the normal control group (lane 1). This confirms that MAPK/ERK pathways in the central pain. CNT LD obviously decreased the protein expression of p-ERK1/2 in corpus striatum (lane 4) which was similar with ZM241385 group (lane 5), whereas the levels of p-ERK1/2 were the highest in DPCPX group (lane 6 and lane 9). Expression of p-ERK1/2 in CNT HD group significantly decreased with treatment of ZM241385 (lane 8). Results suggest that CNT mainly acts on A1Rs by inhibition of p-ERK1/2 to produce analgesic effects, while the hyperalgesia caused by HD CNT is due to activation of A2ARs to enhance p-ERK1/2 expression.
Figure 3.
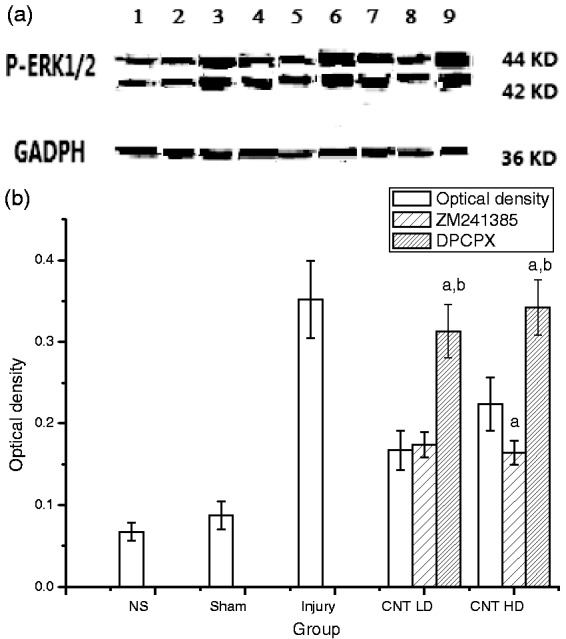
Expression of p-ERK 1/2 in corpus striatum of mice with spinal cord injury. (a) Western blotting, (b) optical density of the bands. Lanes 1 to 9 represent the groups of normal saline (NS), sham, compression injury, CNT LD (25 µg/kg), CNT LD+ZM241385, CNT LD+DPCPX, CNT HD (100 µg/kg), CNT HD+ZM241385, CNT HD+DPCPX, respectively. Data were represented as , n = 8. a, p < 0.05, compared with CNT group in corresponding dose; b, p < 0.05, compared with the group of ZM241385 in corresponding CNT dose.
Discussion
Pain model rationality and gender difference
Rodent pain models are used at all stages from to estimation of efficacy to identification of mechanisms. There are many kinds of acute pain models including acetic acid-induced writhing, formalin-induced inflammation and hot plate test, and chronic pain models including SCI and peripheral nerve injury. But each animal model has shortcomings and limitations,27 two or more animal models should be considered for the validity in clinical application. Unlike other acute pain models, hot plate test is capable of predicting a potency ranking of analgesics corresponding to clinical dosing in postoperative pain.28 It is a common model of thermal-induced nociception used to evaluate central action.29 Therefore, it is a quick and safe method to evaluate central effect of CNT. Peripheral nerve injury models include chronic constriction injury model, partial sciatic nerve ligation model, and spinal nerve ligation, which rely on traumatic injury to a single nerve, but they differ not only in duration and magnitude of sensory changes, signs of spontaneous pain but also in technical difficulty and reproducibility.30 SCI models are typical for central neuropathic pain such as contusion injury, excitotoxic injury, and photochemical injury, etc., among them the contusion injury has been applied in this experiment because of high reproducibility and similarity to human spontaneous pain.23
Only female mice were used in the hot plate test because of the model limitation, but in order to make unbiased results, both male and female mice are better to be used for the SCI model. It is reported that adaptive immune cells, possibly T cells, may mediate pain hypersensitivity in female mice.31 It shows gender differences in pain expression, especially when immune system is involved. But immune-related inflammation is one of the factors inducing pain, the changes in function, chemistry, and structures of neurons (neural plasticity) underlie the production of the altered sensitivity characteristics of neuropathic pain.32 SCI-induced pain are attributed to the nerve loss of central inhibition and the development of behavioral hypersensitivity in a contusive SCI paradigm.33 In our experiments, no significant difference between male and female has been found in the contusion SCI model, suggesting that immune cells are not main pain inducing factors, instead, neurotransmitters plays an important role in pain.
Role of A1Rs and A2ARs in CNT actions
A1Rs and A2ARs have contrary effects on pain: activation of A1Rs causes antinociception, and activation of A2ARs produces pronociception.13 Blocking of A1Rs and A2ARs separately improves CNT’s analgesic and hyperalgesic effects, indicating that CNT takes effects through adenosine receptors. The difference of CNT’s affinity to A1Rs and A2ARs may contribute to its dose effects. CNT has higher affinity to A1Rs than A2ARs, therefore, it shows analgesic effects at lower dose and hyperalgesic effects at higher dose.
Adenosine receptors are widely distributed in central tissues, and corpus striatum is the major site of A1Rs and A2ARs.25 Because spinal cord is the injured tissue relevant to pain signals as reported,34 and hippocampus is the major specific site of CNT.35 The three tissues of spinal cord, corpus striatum, and hippocampus were selected for the detection of ROS and ATP so as to reveal the relationship between CNT and adenosine receptors in pain. There are marked changes of ATP in striatum, further proving the correlation between CNT and A1Rs and A2ARs.
Involvement of ROS and ATP in pain signal pathways and CNT actions
ROS, the metabolic products of aerobic respiration including singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radical (•OH), superoxide anion (O2•−), and lipid peroxide radical (ROO•). etc., mainly distribute in mitochondria36 and could directly damage mitochondria structure resulting in the adenosine accumulation after SCI.37 Preliminary studies denoted that increase of ROS production or decrease of ROS elimination would enhance central algesthesia sensitization.38 ATP is the main energetic material produced in mitochondria for signal transduction process39 and is closely related with adenosine and adenosine receptors.40,41 Therefore, ROS and ATP are upstream signal molecules of adenosine receptor pathway. Our data show that CNT has the effects of adjustment on ROS and ATP, but CNT is not a free radical scavenger and phosphokinase without anti-oxidative groups and phosphate groups in its structure, and cannot interact with ROS and ATP directly. On the other hand, adenosine can mediate its actions through generation of ROS from NADPH oxidase and mitochondrial sources.42 The counterpart of ATP is adenosine, which is produced by breakdown of intra- or extracellular ATP, and adenosine transforming to ATP is helpful for elimination of ROS. CNT might reduce ROS and increase ATP through down regulation of adenosine and improving transformation of adenosine to ATP.
The family of MAPK, a group of serine/threonine protein kinases activated by diverse extracellular stimuli such as hormones and growth factors, etc.,43 are important in the nociception-related processes and plasticity.44 It consists of three separate signal pathways: ERK, p38 MAPK, and c-Jun N-terminal kinase. ERK plays a critical role in neuronal plasticity to nociceptive responses in inflammation and/or nerve injury.45 In several animal models of neuropathic pain, ERK inhibitors could effectively alleviate pain.46 On the other hand, ERK activation by multiple neurotransmitter receptors and different second messengers is involved in central sensitization through regulating the activities of receptors and channels and inducing gene transcription.47 Therefore, the MAPK/ERK pathway could participate in the two ways regulation of CNT process.
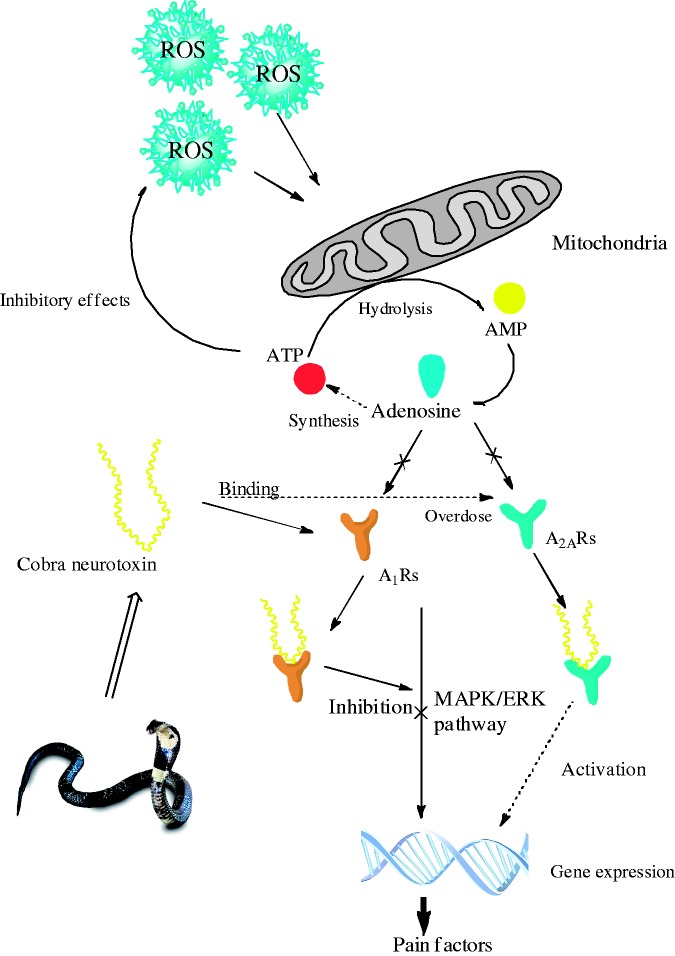
Main mechanism of neuropathic pain is the peripheral sensitization of primary sensory neuron and the central sensitization of spinal dorsal horn, which are regulated by the activation of ERK.48,49 The noxious stimulation activates MAPK/ERK signal pathway via receptors, protein kinases, or ion channels to phosphorylate the substrate proteins and change synaptic plasticity leading to pain sensitization.50 Here, A1Rs activated by CNT competes with noxious stimulation including ROS and adenosine, resulting in the inhibition of ERK phosphorylation and the interruption of pain signals transmission via MAPK/ERK pathway so as to achieve the central analgesia, but excess CNT further activates A2aRs leading to the sensitization. This mechanism was illustrated in Figure 4. The CNT in LD could selectively act on A1Rs rather than A2aRs, possibly because it has stronger binding power to A1Rs.
Figure 4.
The mechanism of analgesic and hyperalgesic effects of cobra neurotoxin.
ROS: reactive oxygen species; ATP: adenosine triphosphate; MAPK: mitogen-activated protein kinases; ERK: extracellular signal-regulated protein kinase.
Conclusion
The central analgesic effects of CNT have been demonstrated by hot plate test in mice and SCI model of mice. LD of CNT improves pain threshold and locomotive behavior, but HD of CNT causes hyperalgesic effects. The action of CNT is related to adenosine receptors pathway. CNT decreases ROS and increases ATP in mice brain tissues leading to reduction of pain inducers; on the other hand, it interrupts the transmission of painful signals via MAPK/ERK pathway through activation of A1Rs by CNT instead of adenosine. But the painful sensitivity is obviously heightened when excessive CNT is administered because of its activation on A2aRs.
Acknowledgments
The authors would like to thank the staff in South China University of Technology for data analysis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported from National Natural Science Funding of China (No. 81173646), Guangzhou and Guangdong Scientific Plan Project (No. 1563000123, No. 2016B090918086).
References
- 1.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets 2005; 9: 699–713. [DOI] [PubMed] [Google Scholar]
- 2.Smits H, Kleef MV, Honig W, et al. Spinal cord stimulation induces c-Fos expression in the dorsal horn in rats with neuropathic pain after partial sciatic nerve injury. Neurosci Lett 2009; 450: 70–73. [DOI] [PubMed] [Google Scholar]
- 3.Staud R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr Rheumatol Rev 2015; 11: 109–115. [DOI] [PubMed] [Google Scholar]
- 4.Chung YC, Tsou MY, Chen HH, et al. Integrative acupoint stimulation to alleviate postoperative pain and morphine-related side effects: a sham-controlled study. Int J Nurs Stud 2014; 51: 370–378. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Huang F, Szymusiak M, et al. Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity. J Pharmacol Exp Ther 2015; 352: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson TA, Milan-Lobo L, Che T, et al. Identification of the first marine-derived opioid receptor “balanced” agonist with a signaling profile that resembles the endorphins. ACS Chem Neurosci 2017; 8: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pla D, Paiva OK, Sanz L, et al. Preclinical efficacy of Australian antivenoms against the venom of the small-eyed snake, Micropechis ikaheka, from Papua New Guinea: an antivenomics and neutralization study. J Proteomics 2014; 110: 198–208. [DOI] [PubMed] [Google Scholar]
- 8.Ye Y, Li Y, Fang F. Opening of brain blood barrier induced by red light and central analgesic improvement of cobra neurotoxin. J Photochem Photobiol B 2014; 134: 16–22. [DOI] [PubMed] [Google Scholar]
- 9.Shi GN, Liu YL, Lin HM, et al. Involvement of cholinergic system in suppression of formalin-induced inflammatory pain by cobratoxin. Acta Pharmacol Sin 2011; 32: 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao QQ, Qian XY, An JX, et al. Rat Model of trigeminal neuralgia using cobra venom mimics the electron microscopy, behavioral, and anticonvulsant drug responses seen in patients. Pain Physician 2015; 18: E1083–E1090. [PubMed] [Google Scholar]
- 11.Larsson M, Broman J. Pathway-specific bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II at spinal nociceptive synapses after acute noxious stimulation. J Neurosci 2006; 26: 4198–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebastião AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol 2009; 193: 471–534. [DOI] [PubMed] [Google Scholar]
- 13.Sawynok J. Adenosine receptor targets for pain. Neuroscience 2016; 338: 1–18. [DOI] [PubMed] [Google Scholar]
- 14.Bura SA, Nadal X, Ledent C, et al. A2a adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain 2008; 140: 95–103. [DOI] [PubMed] [Google Scholar]
- 15.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs 2008; 17: 1901–1910. [DOI] [PubMed] [Google Scholar]
- 16.Thany SH, Courjaret R, Lapied B. Effect of calcium on nicotine-induced current expressed by an atypical alpha-bungarotoxin-insensitive nAChR2. Neurosci Lett 2008; 438: 317–321. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Lin HM, Zou R, et al. Suppression of complete Freund’s adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol Sin 2009; 30: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeusler D, Grassinger L, Fuchshuber F, et al. Hide and seek: a comparative autoradiographic in vitro investigation of the adenosine A3 receptor. Eur J Nucl Med Mol Imaging 2015; 42: 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y. Regulation of cerebral permeation activated by photosensitizers from TCM and analgesia mechanism of peptides from snake venom. Dissertation, South China University of Technology, 2014.
- 20.Hayashi T, Katsuyama S, Orito T, et al. Antinociceptive effect of tebanicline for various noxious stimuli-induced behaviours in mice. Neurosci Lett 2016; 638: 46–50. [DOI] [PubMed] [Google Scholar]
- 21.De P, Dasgupta SC, Gomes A. A lethal neurotoxic protein from Indian king cobra (Ophiophagus hannah) venom. Indian J Exp Biol 2002; 40: 1359–1364. [PubMed] [Google Scholar]
- 22.Guo PJ, Yan RG, Li QB, et al. Effect of ethanol on toxicity of Naja naja atra venom. J Snake 2011; 23: 348–350. [Google Scholar]
- 23.Marques SA, Garcez VF, Del Bel EA, et al. A simple, inexpensive and easily reproducible model of spinal cord injury in mice: morphological and functional assessment. J Neurosci Methods 2009; 177: 183–193. [DOI] [PubMed] [Google Scholar]
- 24.Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23: 635–659. [DOI] [PubMed] [Google Scholar]
- 25.Svenningsson P, Hall H, Sedvall G, et al. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 1997; 27: 322–335. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Cho HY, Ahn YJ, et al. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain 2012; 153: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 27.Nicole EB, Heather LP, Churmy YF, et al. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res 2017; 95: 1242–1256. [DOI] [PubMed] [Google Scholar]
- 28.Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand 1997; 41: 94–111. [DOI] [PubMed] [Google Scholar]
- 29.Ghildiyal S, Gautam MK, Joshi VK, et al. Analgesic and hypnotic activities of Laghupanchamula: a preclinical study. Ayu 2014; 35: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 2005; 80: 93–108. [DOI] [PubMed] [Google Scholar]
- 31.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain 2016; 157: S2–S6. [DOI] [PubMed] [Google Scholar]
- 32.Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12: 304–314. [DOI] [PubMed] [Google Scholar]
- 33.Berrocal YA, Almeida VW, Puentes R, et al. Loss of central inhibition: implications for behavioral hypersensitivity after contusive spinal cord injury in rats. Pain Res Treat 2014; 2014: 178278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis MF, Boyce-Rustay JM. Neuropathic pain: models and mechanisms. Curr Pharm Des 2009; 15: 1711–1716. [DOI] [PubMed] [Google Scholar]
- 35.Davies AR, Hardick DJ, Blagbrough IS, et al. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology 1999; 38: 679–690. [DOI] [PubMed] [Google Scholar]
- 36.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol 2015; 4: 381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao L, Avshalumov MV, Patel JC, et al. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci 2009; 29: 9002–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clausen A, McClanahan T, Ji SG, et al. Mechanisms of rapid reactive oxygen species generation in response to cytosolic Ca2+ or Zn2+ loads in cortical neurons. PLoS One 2013; 8: 1611–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Ma T. Metabolic regulation of mesenchymal stem cell in expansion and therapeutic application. Biotechnol Prog 2015; 31: 468–481. [DOI] [PubMed] [Google Scholar]
- 40.Li DP, Chen SR, Pan HL. Adenosine inhibits paraventricular pre-sympathetic neurons through ATP-dependent potassium channels. J Neurochem 2010; 113: 530–542. [DOI] [PubMed] [Google Scholar]
- 41.Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med 2011; 17: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebremedhin D, Weinberger B, Lourim D, et al. Adenosine can mediate its actions through generation of reactive oxygen species. J Cereb Blood Flow Metab 2010; 30: 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa AP, Lopes MW, Rieger DK, et al. Differential activation of mitogen-activated protein kinases, ERK 1/2, p38(MAPK) and JNK p54/p46 during postnatal development of rat hippocampus. Neurochem Res 2016; 41: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 44.Lee LK, Kim JH, Kim MY, et al. A review of signal transduction of endothelin-1 and mitogen-activated protein kinase-related pain for nanophysiotherapy. J Phys Ther Sci 2014; 26: 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Gereau RW, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao Z, Cao D, Han X, et al. Both JNK and P38 MAPK pathways participate in the protection by dexmedetomidine against isoflurane-induced neuroapoptosis in the hippocampus of neonatal rats. Brain Res Bull 2014; 107: 69–78. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y, Li K, Fu KY, et al. Central sensitization and MAPKs are involved in occlusal interference-induced facial pain in rats. J Pain 2013; 14: 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu J, Song ZY, Zhang HH, et al. Colonic hypersensitivity and sensitization of voltage-gated sodium channels in primary sensory neurons in rats with diabetes. J Neurogastroenterol Motil 2016; 22: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64: 1255–1265. [PubMed] [Google Scholar]
- 50.Imbe H, Senba E, Kimura A, et al. Activation of mitogen-activated protein kinase in descending pain modulatory system. J Signal Transduct 2011; 2011: 468061. [DOI] [PMC free article] [PubMed] [Google Scholar]