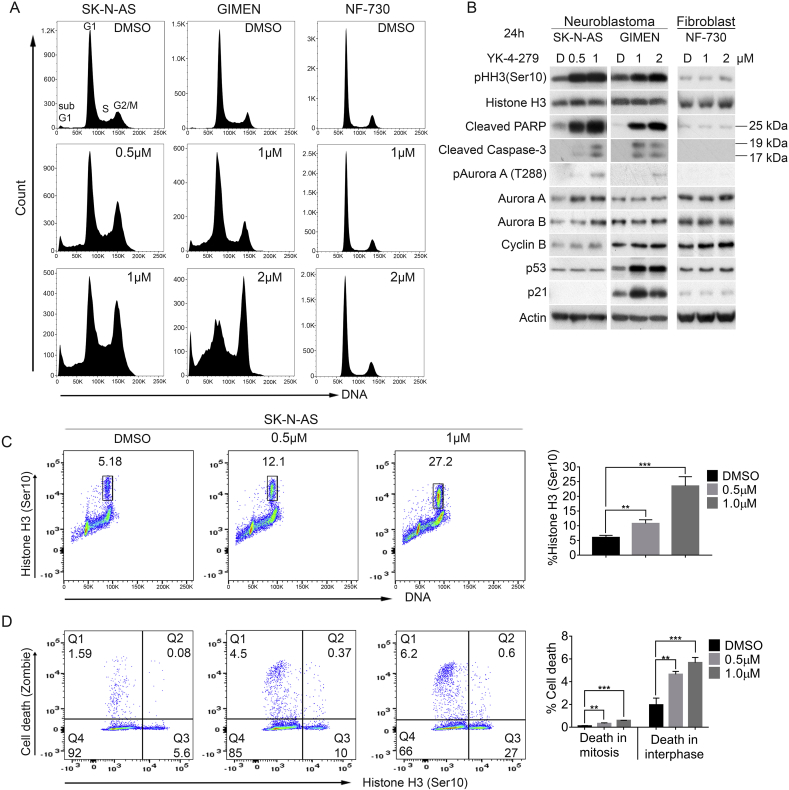
Fig. 3.
YK-4-279 induces mitotic arrest and apoptosis in neuroblastoma cell lines. (A) Propidium-iodide based cell cycle analysis of YK-4-279 treated cells using flow cytometry. Cells were treated for 24 h with 0.5× and 1xIC50 concentrations. Cell cycle analysis was carried out in three independent biological replicates. (B) SK-N-AS, GIMEN and NF-730 were treated with DMSO (D) or two doses of YK-4-279 for 24 h and protein lysates harvested. Blots were probed for several cell cycle and cell death markers. All neuroblastoma cell lines showed increase of phospho-histone H3 (Ser10(pHH3), cleaved PARP and active caspase-3. Actin was used as a loading control. (C) Flow cytometry with triple staining for phospho-histone H3 (Ser10) (mitotic cells). Dot plot showing DNA content (X-axis) against phospho-histone H3 (Y-axis). Quantification bar graphs of percentage mitotic cells (right side). Data shown as mean ± SD of three technical replicates. (D) Zombie live/dead cell marker versus phospho-histone H3(Ser10). Cells synchronized using double-thymidine block were treated with YK-4-279 for 24 h after release. Cells were stained with Zombie dye followed by phospho-histone H3 (Ser10) after fixation and permeabilization and subsequently with propidium iodide. Dot plots of phospho-histone H3 positive cells against dead cells. Q1 and Q2 represent percentage dead cells (Q1 and Q2) negative or positive for phospho-histone H3, respectively. Data shown as mean ± SD of three technical replicates. **P < 0.01, ***P < 0.001. Unpaired Student t-test was used to assess the significance of difference between the control and treated samples.