Figure 2. CNOT2 increases ubiquitination and degradation of p62/SQSTM1 protein in HEK293 QBI cells.
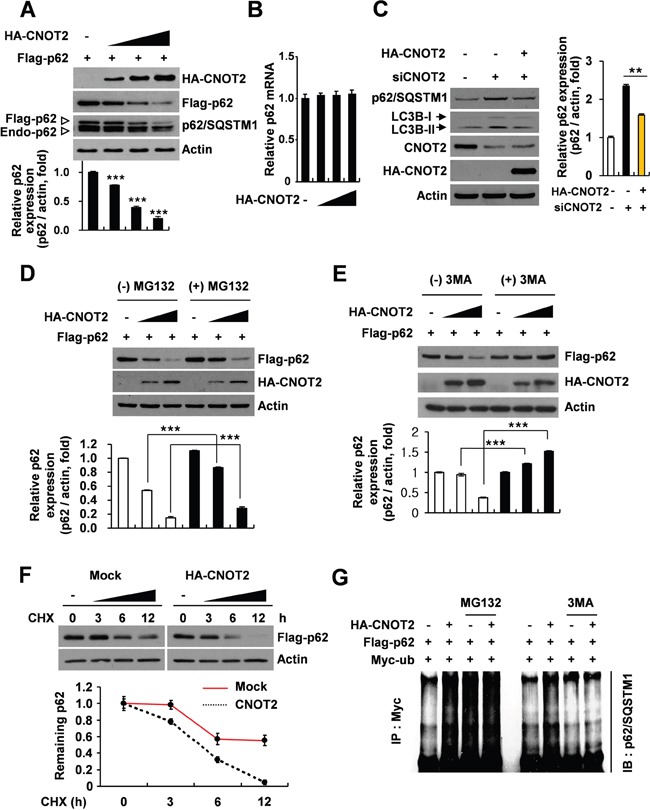
HEK293 QBI cells were co-transfected with Flag-p62 and HA-CNOT2 plasmids. Thereafter, the cells were cultured for 48 h and subjected to (A) western blot or (B) RT-PCR (means ± SD of 3 independent experiments, *** p < 0.001 vs control by Student t test). (C) Cells were transiently transfected with CNOT2 siRNA for 48 h and then subjected to immunoblotting with indicated antibodies (means ± SD of 3 independent experiments, ** p < 0.01 vs control by Student t test). (D) HEK293 QBI cells were transiently transfected with Flag-p62 and HA-CNOT2 plasmids and incubated for 48 h in the presence or absence of MG132 (means ± SD of 3 independent experiments, *** p < 0.001 vs untreated control by Student t test). (E) HEK293 QBI cells were transiently transfected with Flag-p62 and HA-CNOT2 plasmids and incubated for 48 h in the presence or absence of 3-MA. Cell lysates were immunoblotted with indicated antibodies (means ± SD of 3 independent experiments, ** p < 0.01; *** p < 0.001 vs untreated control by Student t test). (F) HEK293 QBI cells were transiently transfected with HA-CNOT2 plasmid or empty vector in the presence or absence of CHX. (G) HEK293 QBI cells were transiently co-transfected with HA-CNOT2, Flag-p62, and Myc-ubiquitin plasmids in the presence or absence of MG132 and 3-MA. Ubiquitination of p62 was evaluated with anti-p62/SQSTM1 antibody.
