Abstract
Resting state functional connectivity studies of tinnitus have provided inconsistent evidence concerning its neural bases. This may be due to differences in the methodology used, but it is also likely related to the heterogeneity of the tinnitus population. In this study, our goal was to identify resting state functional connectivity alterations that consistently appear across tinnitus subgroups. We examined two sources of variability in the subgroups: tinnitus severity and the length of time a person has had chronic tinnitus (referred to as tinnitus duration). Data for the current large-scale analysis of variance originated partly from our earlier investigations (Schmidt et al., 2013; Carpenter-Thompson et al., 2015) and partly from previously unpublished studies. Decreased correlations between seed regions in the default mode network and the precuneus were consistent across individuals with long-term tinnitus (who have had tinnitus for greater than one year), with more bothersome tinnitus demonstrating stronger decreases. In the dorsal attention network, patients with moderately severe tinnitus showed increased correlations between seeds in the network and the precuneus, with this effect also present in only some patients with mild tinnitus. The same effects were not seen in patients with mild tinnitus and tinnitus duration between 6 and 12 months. Our results are promising initial steps towards identifying invariant neural correlates of tinnitus and indexing differences between subgroups.
Keywords: Tinnitus, Resting state functional connectivity, fMRI, Default mode network, Dorsal attention network, Precuneus
Highlights
-
•
Resting state functional connectivity in tinnitus subgroups was examined.
-
•
Default mode network showed decreased correlation to precuneus in long-term tinnitus.
-
•
Dorsal attention network had stronger correlation to precuneus in bothersome tinnitus.
1. Introduction
The investigation of resting state networks via functional magnetic resonance imaging (fMRI) has become widely prevalent in recent years. These networks, which are defined by their unique patterns of spontaneous fluctuations in the blood oxygenation level dependent (BOLD) response, are frequently examined to dissociate patient groups from neurotypical controls and to help illuminate the neurological consequences of disorders, including Alzheimer's disease (Greicius et al., 2004), schizophrenia (Woodward et al., 2011), depression (Mulders et al., 2015), and more (Castellanos et al., 2008, Northoff, 2014). This tool has also been applied to the study of tinnitus, the chronic perception of a sound without an objective source (Husain and Schmidt, 2014).
Despite the emergence of many studies of tinnitus using resting state fMRI (for example, Burton et al., 2012, Maudoux et al., 2012b, Maudoux et al., 2012a, Lanting et al., 2016, Leaver et al., 2016), only general trends of tinnitus' effect on connectivity have been found. As described by Husain and Schmidt (2014), these trends include auditory-limbic interactions, alterations to attention-related networks, and changes in default mode network (DMN) connectivity. Since the publication of the Husain and Schmidt (2014) review, many other studies have been conducted (e.g., Ueyama et al., 2013, Chen et al., 2014, Davies et al., 2014, Han et al., 2014, Lanting et al., 2016, Leaver et al., 2016), and many of them have found results that fit into the suggested categories. More frequently, though, each additional study has provided at least one novel result and often has failed to replicate the findings of older literature.
Two of the more conspicuous examples of discrepancies within the literature include the studies of Kim et al. (2012), Davies et al. (2014), Wineland et al. (2012), and Schmidt et al. (2013). First, in the Kim et al. (2012) study, the authors found lower functional connectivity scores between the left and right primary auditory cortices in tinnitus patients compared to controls, as well as increased functional connectivity between the auditory network and the amygdala. However, another group (Davies et al., 2014), using the same analysis technique and a larger group of participants, was unable to replicate this finding. Schmidt et al. (2013) and Wineland et al. (2012) were also inconsistent. Schmidt et al. (2013) noted several alterations to different resting state networks in non-bothersome tinnitus patients, whose scores on the Tinnitus Handicap Inventory (THI) (Newman et al., 1996) ranged from 0 to 22 (mean 8.33 ± 6.76). Such results included increased connectivity between the auditory network and the parahippocampus, as well as decreased correlations between the DMN and the precuneus, in patients with tinnitus when compared to normal hearing and hearing loss controls. Wineland et al. (2012) used a different analysis technique but a similar population of non-bothersome tinnitus patients (with THI scores ranging from 0 to 24, mean 9.58 ± 6.41), yet found no connectivity alterations in this group compared to the controls.
Differences across studies are influenced in part by the diverse nature of the analysis techniques used. Another element to be considered, however, is the heterogeneity within the tinnitus population. Percepts are incredibly variable, differing in loudness, pitch, timbre, and where the sound is perceived (right ear, left ear, center of head). Additionally, patients within studies often differ in the length of time they had their percept (which we refer to as the tinnitus duration), an important factor when considering possible plasticity and habituation. Perhaps most importantly, the reaction patients have to their tinnitus differs; most patients are not bothered by their percept, but some are very disturbed by it, with strong consequences on quality of life and emotional state (Davis and Rafaie, 2000).
Different subgroups of tinnitus with various combinations of behavioral characteristics could exhibit varying patterns of resting state functional connectivity. We have previously published findings relating to one such trait, tinnitus duration (Carpenter-Thompson et al., 2015). In that study, we compared a group of patients with mild tinnitus who had developed tinnitus recently, having had their percept for > 6 months but less than one year, to another group of patients with mild tinnitus that had their tinnitus for > one year. We examined three resting state networks (the DMN, dorsal attention, and auditory networks) and the only significant result was found in the DMN; patients with long-term tinnitus had decreased connectivity between seed regions and the precuneus when compared to the recent-onset group. This finding echoes the results found by Schmidt et al. (2013), which compared the same long-term group from Carpenter-Thompson et al. (2015) to control groups without tinnitus, and suggests that this disruption to the DMN is not immediate but occurs over time in patients.
In the current study, we examined an additional tinnitus characteristic's effect on resting state connectivity: tinnitus severity. Tinnitus severity in resting state studies has been variable, ranging from mild to catastrophic (Husain and Schmidt, 2014); it could therefore help to explain the differing results. The existing literature seems to suggest that tinnitus severity has a significant impact on the resting state (Burton et al., 2012, Maudoux et al., 2012b, Wineland et al., 2012). However, tinnitus groups with different severities have yet to be directly compared.
Additionally, whereas identifying the differences in resting state functional connectivity between tinnitus subgroups is useful, identifying alterations consistent across subgroups is equally important. Such invariant alterations in connectivity could serve as markers of tinnitus and, eventually, as diagnostic tools. This paper therefore has two goals: first, to identify differences in resting state functional connectivity due to tinnitus severity to supplement our previous findings related to duration, and second, to identify potential markers of tinnitus across varying subgroups by combining participants across studies.
To achieve the first goal, we compared resting state functional connectivity in a group of patients with mild, long-term tinnitus to a group with bothersome, long-term tinnitus. To achieve the second, we also performed a large-scale analysis of variance examining multiple tinnitus subgroups and controls. These groups include the mild, long-term and bothersome, long-term tinnitus groups from the current study, as well as the mild, recent-onset tinnitus group from Carpenter-Thompson et al. (2015), the mild, long-term tinnitus group from Schmidt et al. (2013), and the normal hearing and hearing loss control groups from Schmidt et al. (2013). For both goals, we used the same analysis techniques we have used previously and examined the same three resting state networks: the DMN, the dorsal attention network (DAN), and the auditory network (AUD) (Schmidt et al., 2013, Carpenter-Thompson et al., 2015).
This study therefore builds on our previous work in several key ways. First, we addressed the variable of tinnitus severity by comparing two new groups of tinnitus patients with differing severity scores. The large-scale analysis of variance of the six participant groups across our studies gave us the added benefit of comparing the groups from the current severity analysis to controls. It also allowed us to compare two mild, long-term tinnitus groups collected on different MRI scanners to examine the consistency of resting state functional connectivity in these similar subgroups. Finally, it allowed us to compare the mild, recent-onset group to both the controls from Schmidt et al. (2013), as well as to a group of patients with bothersome tinnitus.
2. Methods
2.1. Participants
Approval for this study was obtained from the University of Illinois at Urbana-Champaign Institutional Review Board (UIUC IRB). Participants were recruited from the Urbana-Champaign area and were scanned under the UIUC IRB 10144 or 12896 protocols. Written informed consent was obtained from each participant and they were suitably compensated.
Six groups of participants were included in this study, including four tinnitus groups. These groups included a subgroup of 13 patients with mild, long-term tinnitus (MLTIN_1; age 55.00 ± 6.97, 3 F); a subgroup of 12 patients with mild, recent-onset tinnitus (MRTIN; age 48.38 ± 12.15, 8 F); a subgroup of 17 patients also with mild, long-term tinnitus (MLTIN_2; age 51.65 ± 11.79, 4 F); and a subgroup of 15 subjects with bothersome, long-term tinnitus (BLTIN; 50.07 ± 10.23, 7 F). The MLTIN groups differ in that data were collected on two different Siemens MRI magnets. Two control groups were also included in the study: one with hearing loss matched to the MLTIN_1 group (HL; 57.62 ± 9.39, 8 F) and another with normal hearing (NH; 53.00 ± 8.73, 6 F) at all testing frequencies. The groups did not significantly differ in age or gender (age: F(5,79) = 1.55, p = 0.18; gender: F(5,79) = 1.65, p = 0.16).
Note that the resting state data for the MLTIN_1, HL and NH groups were analyzed independently of the other groups in a previous paper (Schmidt et al., 2013). A resting state analysis contrasting the MLTIN_1 and MRTIN groups was also previously performed (Carpenter-Thompson et al., 2015). See Table 1 for a summary of demographic information. Please note that tinnitus duration was treated as a categorical variable; participants were labeled as having had tinnitus for > 6 months but < 1 year, or as having had tinnitus for > 1 year. More detailed duration information was only recorded for some participants and is not included here.
Table 1.
Demographic information for all subject groups.
| Group | Age | Gender | Handedness | BAI | BDI-II | Pure tone average (both ears) | THI | Tinnitus duration | Laterality |
|---|---|---|---|---|---|---|---|---|---|
| NH | 53.00 ± 8.73 | 6 F; 9 M | 1 L; 14 R | 1.13 ± 1.30 | 1.60 ± 2.20 | 13.60 ± 8.40 | N/A | N/A | N/A |
| HL | 57.62 ± 9.39 | 8 F; 5 M | 1 L; 12 R | 1.84 ± 1.86 | 3.46 ± 4.41 | 28.54 ± 19.73 | N/A | N/A | N/A |
| MRTIN | 48.38 ± 12.15 | 8 F; 5 M | 1 L; 12 R | 3.62 ± 1.95 | 4.69 ± 2.31 | 17.71 ± 18.93 | 16.46 ± 4.63 | > 6 months, but < 1 year | 3 bilateral; 1 unilateral |
| MLTIN_1 | 55.00 ± 6.97 | 3 F; 9 M | 1 L; 11 R | 1.42 ± 1.73 | 1.25 ± 2.05 | 24.64 ± 15.47 | 8.33 ± 6.76 | > 1 year | 8 bilateral; 2 left |
| MLTIN_2 | 51.65 ± 11.79 | 4 F; 13 M | 1 L; 16 R | 1.53 ± 2.47 | 1.97 ± 2.38 | 21.49 ± 18.45 | 9.41 ± 4.73 | > 1 year | 15 bilateral; 1 left |
| BLTIN | 50.07 ± 10.23 | 7 F; 8 M | 4 L; 11 R | 4.87 ± 3.25 | 5.47 ± 4.29 | 31.07 ± 20.62 | 29.47 ± 10.89 | > 1 year | 13 bilateral; 1 right |
Age, THI, BAI, BDI-II and pure tone averages are written as mean ± standard deviation. The pure tone average is an average across all tested frequencies (0.25, 0.5, 1, 2, 4, 6, 8 kHz) and both ears. For the MRTIN group, PTA is based on data from 6 participants. Laterality information is unavailable for two MLTIN_1 participants, one MLTIN_2 participant, one BLTIN participant, and nine MRTIN participants (due to incomplete audiometric data). Also, for one MRTIN participant, it is unknown whether their unilateral tinnitus was in the left or right ear. F, female; M, male; R, right; L, left; THI, Tinnitus Handicap Inventory; BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory.
To be included in the study, participants were required to be between the ages of 30 and 70, with no hyperacusis as assessed via an in-house questionnaire, loudness discomfort levels, and in the case of the MLTIN_2 and BLTIN groups, the Khalfa Hyperacusis questionnaire (Khalfa et al., 2002). All participants were required to be free of neurological disorders, Meniere's disease, temporomandibular joint disorders, depressive or anxious symptoms, and other neurological issues or chronic physical diseases. No participants were undergoing treatment for tinnitus at the time of study participation. Normal hearing and hearing loss controls were age-, gender-, and in the case of the hearing loss group, hearing threshold-matched to the MLTIN_1 group.
2.2. Measures
Tinnitus severity was classified according to the Tinnitus Handicap Inventory (THI) (Newman et al., 1996). THI scores were collected twice for each participant, both at a hearing assessment and prior to being scanned. All scores listed in this paper are based on the measurement taken on the scan date. The MLTIN_1 group had scores ranging from 0 to 22 (average 8.33, standard deviation 6.76). The MRTIN group had the most variable THI scores, ranging from 0 to 34 (average 16.46, standard deviation 10.17); this was significantly different from the other mild tinnitus groups (with MLTIN_1, t(21) = − 2.37, p = 0.027; with MLTIN_2, t(16) = − 2.32, p = 0.034), but still within the mild clinical category of the THI.
Scores for the MLTIN_2 group were very similar to MLTIN_1, ranging from 0 to 18 (average 8.33, standard deviation 6.76). Note that the MLTIN_1 and MLTIN_2 groups did not significantly differ in THI score (t(18) = 1.73, p = 0.64). The BLTIN group had scores in the “mild” to “moderate” categories of the THI, which include scores in the 18–36 and 38–56 range respectively (range 18–50, average 29.47, standard deviation 10.89). Group THI information is listed in Table 1. A THI score of 18 was the cutoff for deciding whether participants were included in the BLTIN or MLTIN_2 groups. If a participant had a score of exactly 18 on the day of the MRI scan, they were grouped according to their THI score from the day of the hearing test; if the score at the hearing assessment was > 18, they were placed in the BLTIN group, and if the score was < 18, they were placed in the MLTIN_2 group.
Audiological assessments were completed for each subject. Pure tone thresholds were measured at 0.25, 0.5, 1, 2, 4, 6, and 8 kHz. Pure tone averages across both ears and all measured frequencies for all groups are listed in Table 1. Incomplete audiometric data was collected for the MRTIN group as reported in Carpenter-Thompson et al. (2015); the listed pure tone threshold is based on measurements from six participants. Hearing thresholds did not differ significantly between the HL, MLTIN_1, MRTIN, MLTIN_2, and BLTIN groups (F(4, 58) = 1.69, p = 0.16).
Tinnitus was primarily bilateral across all groups; this information can be found in Table 1. Note that this data is unavailable for two MLTIN_1 participants, one MLTIN_2 participant, one BLTIN participant, and nine MRTIN participants. Also, for one MRTIN participant, it is unknown whether their unilateral tinnitus was in the left or right ear.
Depression and anxiety were assessed via the Beck Depression (BDI-II) and Beck Anxiety (BAI) inventories, respectively. All participants indicated scores in the “minimal” clinical category for the BDI-II and were either in the “minimal” or “mild” categories for the BAI. Scores on both questionnaires were significantly different across groups according to a one-way analysis of variance (BDI-II: F(5,79) = 3.71, p = 0.0046; BAI: F(5,79) = 5.68, p = 0.00016). These scores are also listed in Table 1.
2.3. Data acquisition
During resting state scans, subjects were instructed to lay still and fixate on a cross for the duration of the scan, which was continuous and lasted approximately 5 min. All participants were given ear plugs and headphones to wear during the scans. Lights in the scanner room were on, on the second-lowest setting of a 4-level dimmer. The first four images were discarded prior to preprocessing, leaving 146 volumes for analysis.
Data were collected on two different magnets due to decommissioning of one magnet during data collection. The MLTIN_1, MRTIN, NH and HL groups' data were collected on a 3 T Siemens Magentom Allegra MRI head-only scanner. A gradient echo-planar (EPI) sequence with axial orientation was used to collect functional data (repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, flip angle = 90°, 32 slices, voxel size = 3.4 × 3.4 × 4.0 mm3). A high-resolution, T1-weighted, sagittal MPRAGE image was collected (TR = 2300 ms, TE = 2.83 ms, flip angle = 9°, 160 slices, voxel size = 1.0 × 1.0 × 1.2 mm3), as was a T2-weighted, axial TSE image (TR = 7260 ms, TE = 98 ms, flip angle = 150°, 32 slices, voxel size = 0.9 × 0.9 × 4.0 mm3).
The MLTIN_2 and BLTIN groups' data were collected on a 3 T Siemens Magnetom Trio full-body scanner. On this magnet, the EPI sequence used the following parameters: TR = 2000 ms, TE = 25 ms, flip angle = 90°, 38 slices, voxel size = 2.5 × 2.5 × 3.0 mm3. As for the other groups, a low-resolution T2-weighted structural image (TR = 3400 ms, TE = 64.0 ms, flip angle = 120°, 38 slices, voxel size = 1.2 × 1.2 × 3.0 mm3) and a high-resolution T1-weighted MPRAGE (TR = 2300 ms, TE = 3.84 ms, flip angle = 9°, 160 slices, voxel size = 1.0 × 1.0 × 1.2 mm3) were collected.
2.4. Analysis
Preprocessing of the fMRI data was performed using Statistical Parametric Software (SPM8, Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). First, slice-timing correction was performed; scanning was ascending. The images were then realigned to the mean fMRI image using a 6-parameter rigid-body transformation to correct for subject head motion. Two coregistration steps followed the motion correction: a 12-parameter affine transformation was used to align the TSE image to the mean fMRI image, and then, the MPRAGE image was aligned to the TSE image. Next, the MPRAGE image was normalized to an MNI template using a nonlinear warp transformation, which was then also used on the realigned fMRI images. Finally, the normalized, realigned fMRI images were spatially smoothed using a Gaussian kernel of 10 × 10 × 10 mm3.
Following this pre-processing in SPM, seeding analysis was performed using the Functional Connectivity Toolbox (Conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012) for MATLAB. Before executing this analysis, additional pre-processing was done in Conn, including segmentation of the MPRAGE images (which Conn does via SPM8 commands). Time courses associated with white matter and cerebrospinal fluid found as a result of this segmentation and the motion regressors created during the realignment step of preprocessing were regressed out of the data. Additionally, a band-pass filter from 0.008 to 0.08 Hz was applied.
An analysis technique employed in earlier studies (Schmidt et al., 2013, Carpenter-Thompson et al., 2015) was implemented in the current work. Seed-to-voxel analysis was performed to assess functional connectivity. Multiple seed regions were used to represent each network examined. Seeds for the AUD were located in the left and right primary auditory cortices. In the DMN, seeds were placed in the medial prefrontal cortex and posterior cingulate cortex. Finally, in the DAN, seeds were located in the bilateral frontal eye fields and bilateral intraparietal sulci. Seed regions were represented as spheres of 8 mm radius and were created in Marsbar (Brett et al., 2002). The precise coordinates of all seeds are listed in Table 2.
Table 2.
Seed regions used for the resting state connectivity analyses.
| Network | Seed region | Coordinates (MNI) |
||
|---|---|---|---|---|
| x | y | z | ||
| Auditory | Left primary auditory cortex | 55 | − 22 | 9 |
| Right primary auditory cortex | − 41 | − 27 | 6 | |
| Default mode | Medial prefrontal cortex | 8 | 59 | 19 |
| Posterior cingulate cortex | − 2 | − 50 | 25 | |
| Dorsal attention | Left posterior intraparietal sulcus | − 23 | − 70 | 46 |
| Right posterior intraparietal sulcus | 26 | − 62 | 53 | |
| Left frontal eye field | − 25 | − 11 | 54 | |
| Right frontal eye field | 27 | − 11 | 54 | |
Seed regions were 8-mm spheres centered at the listed Montreal Neurological Institute (MNI) coordinates.
Correlation maps of the whole brain were created for each seed for each subject. These individual seed maps were examined together, assessing the main effect of source ROI using a between-sources contrast in Conn. Thus, in the case of the DMN and AUD, the single subject correlation map was a combination of two seeds, and for the DAN, it was a combination of four seeds. These combined correlation maps were z-transformed before group averages were computed to enable across-group comparisons.
First, to directly compare the MLTIN_2 and BLTIN groups, two-sample t-tests were performed in Conn. To be significant, clusters needed to be within a p < 0.025 FDR corrected (for the DMN and auditory networks, Bonferroni corrected for using two seed regions) or p < 0.0125 FDR corrected threshold (for the DAN, Bonferroni corrected for using four seed regions) at either the cluster or voxel level.
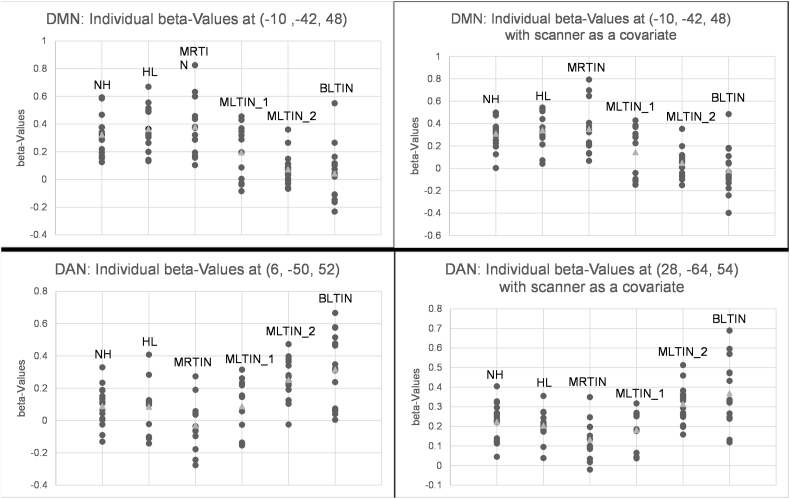
Following this, a one-way analysis of variance (ANOVA) including all six subject groups was conducted in Conn, with significance threshold set at p < 0.025 or p < 0.0125 FDR corrected as appropriate. For the global maximum result in each network, beta-values at significant regions within the ANOVA were also found using the Conn toolbox, which uses REX (Gabrieli Lab, 2013) to extract the mean of the single-subject beta-values within the significant region. The beta-values represent Fisher-transformed correlation coefficient values and are depicted as scatter plots of beta-values vs. subject group (see Fig. 2).
Fig. 2.
The individual beta-values for precuneus connectivity to both default mode and dorsal attention network seeds. The listed MNI coordinates correspond to the peak voxel in the significant clusters, which were the global maxima in the ANOVA analysis. Beta-values from an ANCOVA analysis using scanner as a covariate of no interest are also depicted and were extracted from the peak voxel in the cluster containing the global maxima from the ANOVA analyses. DMN, default mode network; DAN, dorsal attention network.
For the DMN, using scanner as a covariate, the following exploratory post-hoc t-tests were significant at p < 0.025: NH > MLTIN_2, NH > BLTIN, HL > MLTIN_2, HL > BLTIN, MRTIN > MLTIN_2, MRTIN > BLTIN.
For the DAN, using scanner as a covariate, the following exploratory post-hoc t-tests were significant at p < 0.0125: MLTIN_2 > NH, BLTIN > NH, MLTIN_2 > HL, BLTIN > HL, MLTIN_2 > MLTIN_1, BLTIN > MLTIN_1, MLTIN_2 > MRTIN, BLTIN > MRTIN.
An analysis of covariance (ANCOVA) using MRI scanner as a covariate of no interest was also conducted using the Conn toolbox to determine whether the results of the ANOVA analysis were related to differences in scanner. Beta-values from this analysis were extracted for significant differences in clusters that included the global maximum from the ANOVA analysis. Exploratory post-hoc t-tests on the beta-values were performed to assess which groups were driving the main statistical effect. Significance for these tests was set at p < 0.025 for the DMN and p < 0.0125, but were not corrected for the number of comparisons made and therefore should be considered exploratory findings only.
3. Results
3.1. Resting state analysis of tinnitus severity subgroups
No significant differences were found when comparing the MLTIN_2 and BLTIN groups directly in any of the 3 resting state networks examined.
Note that the MLTIN_1 and MRTIN groups were also directly compared to examine the effects of tinnitus duration; this analysis was published previously (Carpenter-Thompson et al., 2015) and was not repeated for this publication. In that study, the only significant finding in the resting state analysis was a significant decrease in the correlation between DMN seeds and the right precuneus in the MLTIN_1 group compared to the MRTIN group.
3.2. Analysis of the auditory network across all groups
No significant differences were found in the ANOVA examining the auditory network across the 6 subject groups.
3.3. Analysis of default mode network across all groups
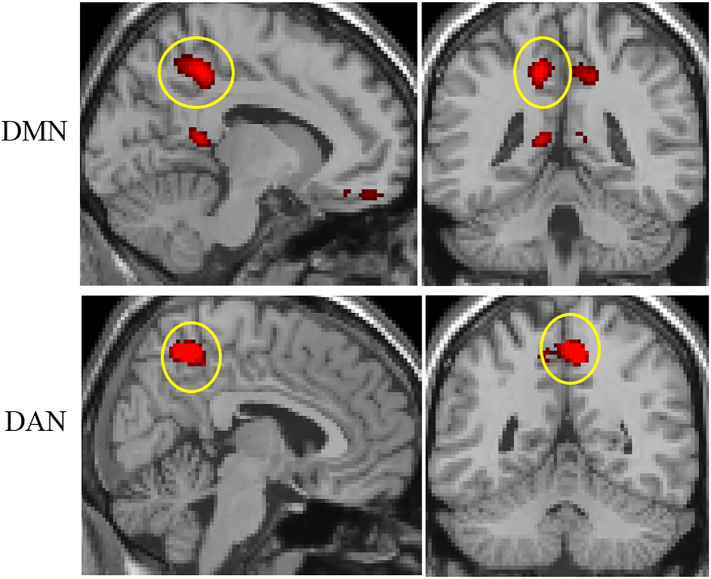
In the ANOVA analysis, significant differences across groups were noted in the precuneus at both the cluster and peak level, as well as in the frontal medial cortex and lateral superior occipital cortex. These results are shown in Supplementary Table 1 and Fig. 1. The beta-value plots for the global maximum in the precuneus, shown in Fig. 2, revealed that the highest average beta-values were noted in the MRTIN group, with the HL and NH controls also showing high values. The BLTIN, MLTIN_1 and MLTIN_2 groups showed distinctly lower beta-values in the precuneus. This observed beta-value pattern remained after the ANCOVA analysis controlling for scanner, as seen in Fig. 2. Exploratory post-hoc t-tests revealed significant differences between the NH group and the MLTIN_2 (t(28) = 5.73, p = 1.90E-06) and BLTIN groups (t(24) = 4.71, p = 8.68E-05); between the HL group and MLTIN_2 (t(21) = 5.34, p = 2.72E-05) and BLTIN (t(26) = 5.03, p = 3.08E-05) groups; and between the MRTIN and MLTIN_2 (t(17) = 4.32, p = 0.000469) and BLTIN (t(24) = 4.41, p = 0.000188) groups. Note that the local maxima in the ANCOVA result did differ slightly from the ANOVA analysis, but not in notable ways; the significant regions did change in cluster size and shifted slightly in some cases, but the overall results did not change.
Fig. 1.
Results for the one-way ANOVAs of both the default mode and dorsal attention networks. The global maxima for each network (circled in yellow) was located in the precuneus. In the default mode, the depicted results are significant at an FDR-corrected threshold of p < 0.025 at both the cluster and peak level. For the DAN, the results are significant at p < 0.0125 FDR-corrected at the cluster level only. DMN, default mode network; DAN, dorsal attention network.
3.4. Analysis of dorsal attention network across all groups
In the DAN, ANOVA analysis revealed a significant difference at two different clusters in the precuneus, at the cluster level only. The results are depicted in Fig. 1 and listed in Supplementary Table 2. The beta-values extracted from the global maximum, depicted in Fig. 2, revealed that the BLTIN group demonstrated the highest average beta-values, followed by the MLTIN_2 group. The MRTIN group showed the lowest values. After the ANCOVA analysis with scanner as a covariate of no interest, the significant region in the precuneus grew larger, with the local maximum of the region shifting and the two clusters from the ANOVA analysis joining together. Additional significant regions in the precentral gyri and the cerebellum were also observed when correcting for scanner. The beta-value plot centered at this new global maximum demonstrated the same pattern as the plot in the ANOVA analysis. Exploratory post-hoc t-test of these beta-values revealed significant differences between the NH and MLTIN_2 (t(29) = − 2.67, p = 0.0122) and BLTIN (t(23) = − 2.92, p = 0.00775) groups; between the HL and MLTIN_2 (t(28) = − 3.53, p = 0.00146) and BLTIN (t(21) = − 3.44, p = 0.00248) groups; between the MLTIN_1 and MLTIN_2 (t(22) = − 3.52, p = 0.00195) and BLTIN (t(24) = − 3.57, p = 0.00154) groups; and between the MRTIN patients with the MLTIN_2 (t(25) = − 5.11, p = 2.81E-05) and BLTIN (t(23) = − 4.64, p = 0.000115) groups.
Because the beta-value plots of the DMN and DAN appeared to demonstrate an inverse relationship between the correlation patterns across subject groups, a Pearson correlation coefficient was calculated comparing DMN-precuneus beta-values to DAN-precuneus beta values. The resulting correlation coefficient was − 0.586.
4. Discussion
The aim of this study was to identify differences in resting state functional connectivity patterns that could serve as potential markers of tinnitus across somewhat heterogeneous subgroups. More specifically, we focused on the default mode, auditory and dorsal attention networks, which have been shown to differ between tinnitus and controls and potentially vary across subgroups. A distinct pattern of altered functional connectivity between seeds in the DMN and the precuneus, as well as between seeds in the DAN and the precuneus, was observed, signaling that the connectivity of the precuneus could serve as a marker for long duration tinnitus. Further, the strength of the correlations between the precuneus and these networks could index tinnitus severity.
In the DMN, the BLTIN group exhibited significantly reduced connectivity between the seeds and the precuneus when compared to controls and patients with mild, recent onset tinnitus (MRTIN), both with and without the use of scanner as a covariate of no interest. The MRTIN group appeared to resemble controls, with beta-values in similar ranges as both the normal hearing and hearing loss controls. Tinnitus did not need to be bothersome to see the effect; the MLTIN_2 group showed reduced connectivity to the precuneus when compared to both MRTIN and controls. In the DAN, the patterns were partially reversed, with the BLTIN group showing increased connectivity between the seeds and the precuneus when compared to controls, MRTIN, and MLTIN_1 groups. The MLTIN_2 group also showed increased connectivity to the precuneus when compared to the MRTIN group. The MLTIN_1 group did not show these patterns when compared to controls. Again, this pattern held regardless of whether or not scanner was used as a covariate of no interest. Overall, these results suggest that disrupted connectivity between the precuneus and other default mode regions could be an indicator of long-term tinnitus, with the strength of the disruption correlated with tinnitus severity. Additionally, this reduced connectivity in the DMN is coupled with increased connectivity between the DAN and the precuneus in patients with long-term bothersome tinnitus, though whether it occurs in mild, long-term tinnitus may be variable.
As the precuneus is a hub of the DMN (Buckner et al., 2008), it is not unreasonable to suspect that the alterations in its connectivity could interfere with proper function of the network. An important characteristic of the DMN function involves its relationship with the DAN (Fox et al., 2005). The DAN demonstrates anticorrelation with the DMN in fixation, eyes open, and eyes closed rest (Fox et al., 2005). Though initially controversial due to the use of global signal regression in data analysis, the anticorrelated behavior of the networks does hold when global signal regression is not used (for example, Spreng et al., 2016). This relationship has led some to speculate that the brain moves between two distinct, competing processing states, those associated with external (associated with the DAN) and internal processing (associated with the DMN) (Buckner et al., 2008). Importantly, the proper suppression of the DMN has been shown to have an impact on cognition (Spreng and Schacter, 2012). For example, Hampson et al. (2010) showed that correlations between a region in the DMN and DAN were negatively correlated to performance on a working memory task. Whitfield-Gabrieli and Ford (2012), in their review of DMN activity and connectivity, noted that greater suppression of the DMN (and therefore activation of the DAN) is correlated with better memory formation, and that the more difficult a task is, the greater the network suppression. Thus, disrupted cognitive function in tinnitus patients (for example, Andersson and McKenna, 2006) could be partially attributed to the underlying resting state disruptions seen here.
4.1. Expanding on a model of habituation
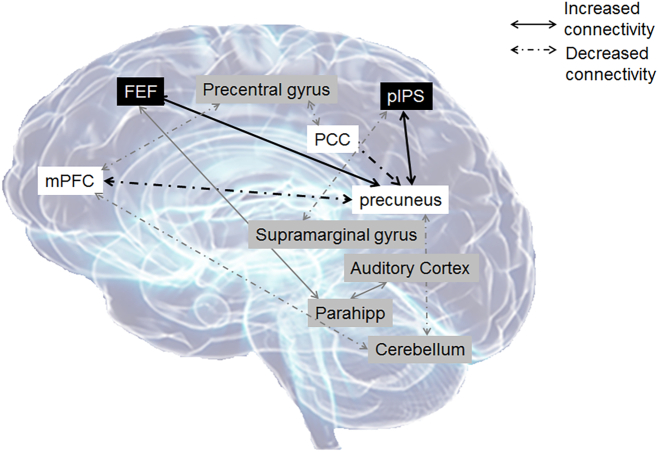
The results of our study also relate to tinnitus habituation. An underlying assumption is that habituation is inversely related to severity, in that those habituated to tinnitus exhibit mild symptoms and those not habituated report bothersome tinnitus. In a recent review (Husain, 2016, Fig. 1), we graphically depicted the effects of mild tinnitus on resting state functional connectivity and hypothesized different patterns of activation and connectivity for habituation. In the current paper, we expand upon this model, and propose a relationship between DAN, DMN and tinnitus severity (see Fig. 3). We propose that as tinnitus severity increases, the spontaneous fluctuations of the precuneus begin to switch from their usual patterns (which results in strong correlations with other regions in the DMN) to more closely resemble the patterns found in the DAN. This effect in the DMN also seems to be a continuous variable, i.e. tinnitus severity places participants along a continuum of precuneus correlation. The continuum is implied by the beta-value plots for the DMN (see Fig. 2). This finding has possible applications to the evaluation of the efficacy of tinnitus interventions. In the context of these results, an effective tinnitus treatment would result in increased connectivity between the precuneus and the DMN and decreased connectivity between the precuneus in the DAN, thus returning to a control-like state. Longitudinal studies would be required to fully understand the relationship between tinnitus severity, habituation, and resting state functional connectivity.
Fig. 3.
A modification of Fig. 1 from Husain, 2016. An illustration of the effect of tinnitus on resting state functional connectivity, compared to controls. The black boxes correspond to regions of the dorsal attention network, while the white boxes are regions associated with the default mode network. In tinnitus patients, connectivity between the precuneus and the other regions of the default mode is decreased. This effect is more pronounced as tinnitus severity increases. Connectivity between the precuneus and regions in the dorsal attention network are increased in patients with bothersome tinnitus, but the effect is inconsistent in patients with mild tinnitus. The gray boxes and lines indicate findings that were noted previously, but were not replicated in the current study. FEF, frontal eye fields; pIPS, posterior intraparietal sulci; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; parahipp, parahippocampus.
The precuneus has also been shown to be related to conscious and internal awareness (Cavanna and Trimble, 2006, Cavanna, 2007, Vanhaudenhuyse et al., 2011) and thus may play a role in tinnitus generation and persistence. Some hypotheses suggest that conscious awareness is necessary for tinnitus perception Even though tinnitus has reached conscious awareness, often if distractors are provided, the percept can be effectively ignored (Roberts et al., 2013). Tinnitus habituation may therefore be linked to networks of awareness, of which the precuneus plays a key role. Future work should include a more careful examination of such networks.
4.2. Our findings and other tinnitus literature
Connectivity differences associated with the precuneus have been noted in other tinnitus studies. A disruption of the DMN was also noted by Lanting et al. (2016), who noted decreased DMN strength in patients. Additionally, Maudoux et al. (2012b), in their assessment of the auditory network, noted that the connectivity of posterior cingulate and precuneus to several brain regions of interest was positively correlated with severity, as indexed by the THI score. Our study also ascribes a relationship between the patterns of precuneus connectivity and tinnitus severity, although it more specifically parses this association to decreased correlations to the DMN and increased correlations to the DAN. Mohan et al. (2016), who probed the resting sate via EEG, also mention the precuneus as belonging to a distress network which includes it as well as the insula and pregenual, subgenual and dorsal anterior cingulate cortex.
However, the results of this study do disagree with other aspects of the literature. Specifically, the differences we note between the non-bothersome tinnitus groups and controls (as in Schmidt et al., 2013) do not correspond to work by Wineland et al. (2012), where no differences between controls and non-bothersome tinnitus were found. Additionally, though previous work (Kim et al., 2012, Maudoux et al., 2012a), noticed altered functional connectivity between auditory and limbic brain regions, in our analysis across tinnitus subgroups we found no significant connectivity differences in the auditory network. It could be that inter-subject variability may be higher when assessing the AUD; there may still be individuals within each group for whom this network is more affected. Particular connectivity changes may emerge only when specific conditions are met, such as in the presence of a task (as in Carpenter-Thompson et al., 2014) or in possession of a certain combination of tinnitus characteristics.
4.3. Caveats
Our conclusions thus far have relied on group-level results. However, an important aspect of studying tinnitus is accounting for individual variability, which may in turn influence intra- and inter-group variability. Examining Fig. 2, which contains the individual beta-values of the precuneus' correlation with network seeds, reveals large variability in all subject groups in both the DMN and DAN. This individual variability makes it difficult to generalize our results to the broader tinnitus population and is important to keep in mind, in particular in relation to discussion of tinnitus interventions. The proposed relationship between the precuneus, the DMN and the DAN in tinnitus patients may be driven specifically by group-level results.
Disruptions of the DMN are not exclusive to tinnitus; in fact, the DMN plays a role in two key factors related to the subject population included in this study. First, the DMN has frequently been examined in the context of aging, due primarily to studies of Alzheimer's disease that have revealed DMN disruption (Andrews-Hanna et al., 2007, Buckner et al., 2008, Sala-Llonch et al., 2015). Andrews-Hanna et al. (2007) noted a DMN disruption primarily in anterior-to-posterior connections within the network, and further, found disruptions within the DAN as well. Note that in our study, ages were not significantly different across groups, and a subsequent analysis using age and gender as covariates of no interest did not impact results. Because of this, it is unlikely that our results can be attributed to age, but it is important caveat to be aware of in both the context of tinnitus and in a broader study of the DMN.
Second, the DMN has also been shown to be altered in patients with major depressive disorder, a condition that is commonly comorbid with bothersome tinnitus (Andersson, 2002). In general, results from studies of the DMN in depression patients indicate a hyperactive network with increased functional connectivity, and it has been suggested that these results relate to the process of rumination (Zhang et al., 2016). For a detailed review of resting state studies of depression, see the paper by Dutta et al. (2014). As indicated in the Methods section of this paper, measures of depression and anxiety (the Beck Depression Inventory, or BDI-II and Beck Anxiety Inventory, or BAI, respectively) were significantly different across groups. This may have had an impact on our results, though the distribution of the scores do not seem to be related to our findings in a clear way. The MRTIN and BLTIN groups had the highest average BAI scores, and the HL, MRTIN and BLTIN groups had higher average BDI-II scores However, as the MRTIN group and BLTIN group demonstrated opposite patterns in precuneus correlations but similar anxiety and depression measures, we would attribute our findings to tinnitus characteristics. Further, our main result of a disrupted DMN in patients with tinnitus is the opposite of what is typically noted in patients with major depressive disorder.
It is also important to note that the results in the DAN were significant only at the cluster-level. Eklund et al. (2016) recently raised important concerns regarding the validity of cluster-level results found using SPM, which should be taken into consideration when interpreting our findings. The DMN results, which were significant at cluster and peak levels, are not affected by this concern.
4.4. Future directions
Though we did conduct an ANCOVA analysis controlling for the scanner used to verify the validity of the beta-plots in each network, the fact that the participants were collected on two MRI scanners remains a concern. It is encouraging that our results do hold when scanner is used as a covariate, both for the validity of our results and for the feasibility of multi-site, multi-scanner studies of tinnitus. Additionally, including the use of different scanners lends itself to our goal of finding invariant markers of tinnitus (which should also be invariant across scanners). A future study with a large sample size and multiple subgroups, but using one scanner, is necessary to replicate our findings.
It is also important to note that this study does not include participants with THI scores above 50. We expect that in patients with more bothersome tinnitus, as indexed by higher THI scores, the pattern seen in the current study would continue, but the differences with the controls or mild tinnitus groups would be amplified - the precuneus would be even more decoupled from the DMN and more correlated with the DAN. Additionally, there are two concerns relating to the MRTIN group worth noting. First, we defined our recent-onset group as having had tinnitus for between 6 months and one year, but the long-term tinnitus groups had tinnitus ranging from 2 to 40 years. Subdividing these long-term groups' duration or including tinnitus duration as a precise, continuous variable could provide more insight into patterns of resting state connectivity. Second, the intersection of tinnitus duration and severity needs to be explored in more depth. Our current evidence suggests that changes in precuneus connectivity is a long-term effect and therefore a consequence (not a cause) of having tinnitus. While we did have a mix of tinnitus severities in the MRTIN group, ranging from THI scores of 0 to 34, directly contrasting a recent-onset, bothersome group of patients to a recent-onset mild group would be needed to verify our findings.
Currently, we also cannot make any inferences regarding effective connectivity between the two networks and the precuneus; structural equation modeling of this data would provide more insight into the connections that facilitate this change. Additionally, it is important to note that the precuneus has been discussed in terms of different subdivisions (Cavanna and Trimble, 2006, Cavanna, 2007). Future work examining the precuneus in tinnitus should consider dissociating between these subdivisions to gain more insight as to the functional consequences of the altered connectivity.
When performing resting state fMRI studies, it is always important to note that there is scanner noise during data collection. This is particularly relevant when studying tinnitus patients, as the sounds from the scanner can have variable effects on tinnitus perception. In some cases, the tinnitus sound may be masked completely; in others, the scanner noise may exacerbate the sound. This confound may explain the different results involving the MLTIN_1 and MLTIN_2 groups found in the DAN. It is possible that the MLTIN_2 group could perceive their tinnitus during the scan while the MLTIN_1 group primarily did not, though this is purely speculation; information regarding the effect of scanner noise on tinnitus masking was only gathered for the MLTIN_1 group, and of them, only 4 participants could not hear their tinnitus while being scanned. We therefore cannot discuss this issue in depth in the current work.
In addition to the effect of scanner noise on tinnitus perception, all participants could have different experiences while in the MRI scanner. While all participants did observe a fixation cross for the duration of the resting state scans, the thought patterns or mind-wandering they experienced during the scans has a strong amount of individual variability that could influence our results (Tusche et al., 2014, Marchetti et al., 2015), in particular in the DMN (Kucyi et al., 2016). Such factors could, for example, help to explain the differences between the MLTIN_1 and MLTIN_2 groups. Future resting state studies of tinnitus should incorporate tools that attempt to examine the types of thinking occurring during resting state scans, such as the Amsterdam Resting-State Questionnaire (Diaz et al., 2013, Diaz et al., 2014). A group of European tinnitus researchers known as TINNET (http://tinnet.tinnitusresearch.net/) has also been developing a questionnaire based on the Amsterdam Resting-State Questionnaire that incorporates questions relating to tinnitus, called the TINNET Resting-state Questionnaire, which could be used. Incorporating physiological data, such as heart rate and respiratory measures, can also provide useful information regarding arousal.
5. Conclusion
Our study begins the work of identifying invariant neural correlates of tinnitus across different subgroups, as well as determining measures that are unique to particular subgroups, using resting state functional connectivity. We propose that DMN-precuneus decoupling is a potential marker of long-term tinnitus, though increased tinnitus severity exaggerates this disruption. Further, coupling of the precuneus and DAN at rest is associated with bothersome tinnitus and is not always observed in those with mild tinnitus, possibly due to differences in habituation or more specific facets of tinnitus severity. Mild, recent-onset tinnitus appears to be a unique subgroup of tinnitus patients where resting state functional connectivity patterns have not yet changed from those seen in controls. The results of our study suggest that the efficacy of intervention studies can be investigated by noting changes in the pattern of DMN-precuneus and the DAN-precuneus connectivity; we hypothesize improvement in measures of tinnitus severity would be correlated with increased DMN-precuneus connectivity.
Author contributions
Conceptualization, F.T.H and S.A.S.; Methodology, F.T.H., S.A.S. and J.C.T.; Formal Analysis, S.A.S.; Investigation, S.A.S. and J.C.T.; Writing—Original Draft, S.A.S. and F.T.H.; Writing—Review and Editing, S.A.S., F.T.H. and J.C.T.; Funding Acquisition, F.T.H. and J.C.T.; Supervision, F.T.H.
Funding
The work was supported by a grant from the Tinnitus Research Consortium (to F.T.H.), the American Tinnitus Association (#392 to J.C.T.), and the Center on Health, Aging and Disability at the University of Illinois (to J.C.T.). Additional support was provided to S.A.S. and J.C.T. from the Neuroengineering National Science Foundation IGERT (Integrative Graduate Education and Research Traineeship), NSF Grant No. 0903622.
Acknowledgments
The authors wish to thank Jaclyn Jansen, AuD and Jenise Chappell, AuD for assistance with audiological assessments and the staff at the Biomedical Imaging Center at the Beckman Institute, University of Illinois for their assistance during MRI data acquisition. We also thank Dr. Ben Zimmerman for his helpful comments on this manuscript. We are grateful to the volunteers who participated in this research.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.07.015.
Appendix A. Supplementary data
Supplementary tables depict the results of the ANOVA analysis of the Default Mode and Dorsal Attention networks, and contrast all six participant groups. Figure 1 contains the visual depictions of these results.
References
- Andersson G. Psychological aspects of tinnitus and the application of cognitive-behavioral therapy. Clin. Psychol. Rev. 2002;22:977–990. doi: 10.1016/s0272-7358(01)00124-6. [DOI] [PubMed] [Google Scholar]
- Andersson G., McKenna L. The role of cognition in tinnitus. Acta Otolaryngol. Suppl. 2006:39–43. doi: 10.1080/03655230600895226. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Snyder A.Z., Vincent J.L., Lustig C., Head D., Raichle M.E., Buckner R.L. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. Vol 16, No 2. 2002. Region of Interest Analysis Using an SPM Toolbox [Abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain. June 2–6, Sendai, Japan. Available on CD-ROM in NeuroImage. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burton H., Wineland A., Bhattacharya M., Nicklaus J., Garcia K.S., Piccirillo J.F. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012;13:3. doi: 10.1186/1471-2202-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Thompson J.R., Akrofi K., Schmidt S.A., Dolcos F., Husain F.T. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res. 2014;1567:28–41. doi: 10.1016/j.brainres.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Carpenter-Thompson J.R., Schmidt S.A., Husain F.T. Neural plasticity of mild tinnitus: an fMRI investigation comparing those recently diagnosed with tinnitus to those that had tinnitus for a long period of time. Neural Plast. 2015;2015:161478. doi: 10.1155/2015/161478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B., Sonuga-Barke E.J., Rotrosen J., Adler L.A., Milham M.P. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E. The precuneus and consciousness. CNS Spectr. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Zhang J., Li X.W., Xia W., Feng X., Gao B., Ju S.H., Wang J., Salvi R., Teng G.J. Aberrant spontaneous brain activity in chronic tinnitus patients revealed by resting-state functional MRI. Neuroimage Clin. 2014;6:222–228. doi: 10.1016/j.nicl.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Gander P.E., Andrews M., Hall D.A. Auditory network connectivity in tinnitus patients: a resting-state fMRI study. Int. J. Audiol. 2014;53:192–198. doi: 10.3109/14992027.2013.846482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A., Rafaie E.A. Epidemiology of tinnitus. In: Tyler R.S., editor. Tinnitus Handbook. San Diego (CA); Singular: 2000. [Google Scholar]
- Diaz B.A., Van Der Sluis S., Moens S., Benjamins J.S., Migliorati F., Stoffers D., Den Braber A., Poil S.S., Hardstone R., Van't Ent D., Boomsma D.I., De Geus E., Mansvelder H.D., Van Someren E.J., Linkenkaer-Hansen K. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Front. Hum. Neurosci. 2013;7:446. doi: 10.3389/fnhum.2013.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B.A., Van Der Sluis S., Benjamins J.S., Stoffers D., Hardstone R., Mansvelder H.D., Van Someren E.J., Linkenkaer-Hansen K. The ARSQ 2.0 reveals age and personality effects on mind-wandering experiences. Front. Psychol. 2014;5:271. doi: 10.3389/fpsyg.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.F. Resting state networks in major depressive disorder. Psychiatry Res. 2014;224:139–151. doi: 10.1016/j.pscychresns.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli Lab . REX: Region of Interest Extraction Toolbox. 2013. McGovern Institute for Brain Research Massachusetts Institute of Technology. [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N., Roth J.K., Gore J.C., Constable R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Zhaohui L., Fei Y., Ting L., Pengfei Z., Wang D., Cheng D., Pengde G., Xiaoyi H., Xiao W., Rui L., Zhenchang W. Abnormal baseline brain activity in patients with pulsatile tinnitus: a resting-state FMRI study. Neural Plast. 2014;2014:549162. doi: 10.1155/2014/549162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain F.T. Neural networks of tinnitus in humans: elucidating severity and habituation. Hear. Res. 2016;334:37–48. doi: 10.1016/j.heares.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Husain F.T., Schmidt S.A. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 2014;307:153–162. doi: 10.1016/j.heares.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Khalfa S., Dubal S., Veuillet E., Perez-Diaz F., Jouvent R., Collet L. Psychometric normalization of a hyperacusis questionnaire. ORL J. Otorhinolaryngol. Relat. Spec. 2002;64:436–442. doi: 10.1159/000067570. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Kim Y.H., Lee S., Seo J.H., Song H.J., Cho J.H., Chang Y. Alteration of functional connectivity in tinnitus brain revealed by resting-state fMRI? A pilot study. Int. J. Audiol. 2012;51:413–417. doi: 10.3109/14992027.2011.652677. [DOI] [PubMed] [Google Scholar]
- Kucyi A., Esterman M., Riley C.S., Valera E.M. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proc. Natl. Acad. Sci. U. S. A. 2016;113:13899–13904. doi: 10.1073/pnas.1611743113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting C., Wozniak A., van Dijk P., Langers D.R. Tinnitus- and task-related differences in resting-state networks. Adv. Exp. Med. Biol. 2016;894:175–187. doi: 10.1007/978-3-319-25474-6_19. [DOI] [PubMed] [Google Scholar]
- Leaver A.M., Turesky T.K., Seydell-Greenwald A., Morgan S., Kim H.J., Rauschecker J.P. Intrinsic network activity in tinnitus investigated using functional MRI. Hum. Brain Mapp. 2016;37:2717–2735. doi: 10.1002/hbm.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A., Baglio F., Costantini I., Dipasquale O., Savazzi F., Nemni R., Sangiuliano Intra F., Tagliabue S., Valle A., Massaro D., Castelli I. Theory of mind and the whole brain functional connectivity: behavioral and neural evidences with the Amsterdam resting state questionnaire. Front. Psychol. 2015;6:1855. doi: 10.3389/fpsyg.2015.01855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A., Lefebvre P., Cabay J.E., Demertzi A., Vanhaudenhuyse A., Laureys S., Soddu A. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A., Lefebvre P., Cabay J.E., Demertzi A., Vanhaudenhuyse A., Laureys S., Soddu A. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012;1485:10–21. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Mohan A., De Ridder D., Vanneste S. Emerging hubs in phantom perception connectomics. Neuroimage Clin. 2016;11:181–194. doi: 10.1016/j.nicl.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Newman C.W., Jacobson G.P., Spitzer J.B. Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Northoff G. Are auditory hallucinations related to the brain's resting state activity? A ‘neurophenomenal resting state hypothesis’. Clin. Psychopharmacol. Neurosci. 2014;12:189–195. doi: 10.9758/cpn.2014.12.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.E., Husain F.T., Eggermont J.J. Role of attention in the generation and modulation of tinnitus. Neurosci. Biobehav. Rev. 2013;37:1754–1773. doi: 10.1016/j.neubiorev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R., Bartres-Faz D., Junque C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front. Psychol. 2015;6:663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.A., Akrofi K., Carpenter-Thompson J.R., Husain F.T. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Schacter D.L. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb. Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TINNET. tinnet. tinnitusresearch.net (accessed 13.07.17).
- Tusche A., Smallwood J., Bernhardt B.C., Singer T. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. NeuroImage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Donishi T., Ukai S., Ikeda Y., Hotomi M., Yamanaka N., Shinosaki K., Terada M., Kaneoke Y. Brain regions responsible for tinnitus distress and loudness: a resting-state FMRI study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A., Demertzi A., Schabus M., Noirhomme Q., Bredart S., Boly M., Phillips C., Soddu A., Luxen A., Moonen G., Laureys S. Two distinct neuronal networks mediate the awareness of environment and of self. J. Cogn. Neurosci. 2011;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Centre for Neuroimaging. Statistical Parametric Mapping 8. http://www.fil.ion.ucl.ac.uk/spm/software/spm8/ (accessed 13.07.17).
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wineland A.M., Burton H., Piccirillo J. Functional connectivity networks in nonbothersome tinnitus. Otolaryngol. Head Neck Surg. 2012;147:900–906. doi: 10.1177/0194599812451414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhu Y., Zhu Y., Wu S., Liu H., Zhang W., Xu C., Zhang H., Hayashi T., Tian M. Molecular, functional, and structural imaging of major depressive disorder. Neurosci. Bull. 2016;32:273–285. doi: 10.1007/s12264-016-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables depict the results of the ANOVA analysis of the Default Mode and Dorsal Attention networks, and contrast all six participant groups. Figure 1 contains the visual depictions of these results.