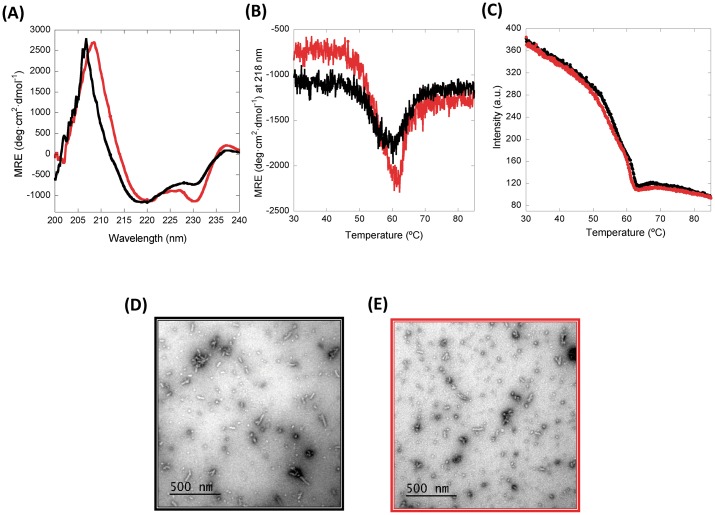
Fig 4. Protein characterization.
(A) Circular Dichroism (CD) spectra at 25°C; (B)Thermal denaturation followed by CD; (C) Thermal denaturation followed by Trp-fluorescence; (D) and (E) TEM micrographs. Black: scFv-h3D6-Ec, Red: scFv-h3D6-Pp. CD analysis showed that the β-conformation characteristic of the immunoglobulin fold is maintained, albeit some differences in the interferences due to the Trp residues in the core of each domain are somehow higher in the scFv-h3D6-Pp spectrum. However, no differences in terms of thermal stability were observed and, therefore, it can be assumed that both molecules are equally folded. As expected, worm-like fibrils, behind the protective effect of scFv-h3D6, are formed upon thermal denaturation in both cases, so that the therapeutic effect should remain.