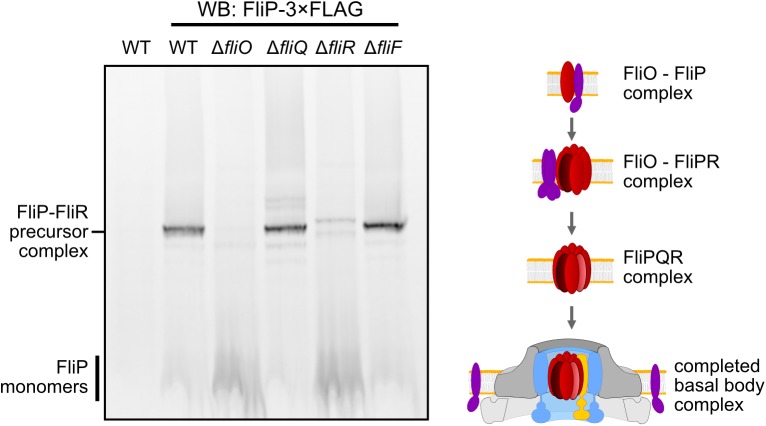
Fig 8. Assembly of FliP subassemblies in core export apparatus mutants and model of the coordinated assembly of the flagellar-specific type III secretion system (fT3SS).
Left: The assembly of stable FliP subassemblies is dependent on FliO and FliR but not on FliQ or FliF. Anti-FLAG Western blot of blue native PAGE (BN-PAGE) of crude membrane extracts prepared from the wild-type (WT) harboring untagged FliP (LT2, TH437) and mutant strains encoding for chromosomal FliP-3×FLAG: WT (EM6221), ΔfliO (EM6222), ΔfliQ (EM6223), ΔfliR (EM6224), ΔfliF (EM4859). Strains EM6221, EM6222, EM6223, and EM6224 additionally harbored a deletion of the proximal rod components flgBC in order to arrest flagellar synthesis after assembly of the core export apparatus. Right: Model of the coordinated assembly of the core flagellar export apparatus. Upon initiation of flagellum assembly, the flagellar type III secretion system (T3SS)-specific chaperone FliO facilitates formation of an oligomeric complex containing FliP and FliR. FliO then presumably dissociates from the stable FliP–FliR core complex. The FliP–FliR core complex forms the nucleus for the assembly of FliQ, FlhB, and FlhA [11], followed by MS-ring (FliF) polymerization and formation of the completed protein export-competent flagellar T3SS.