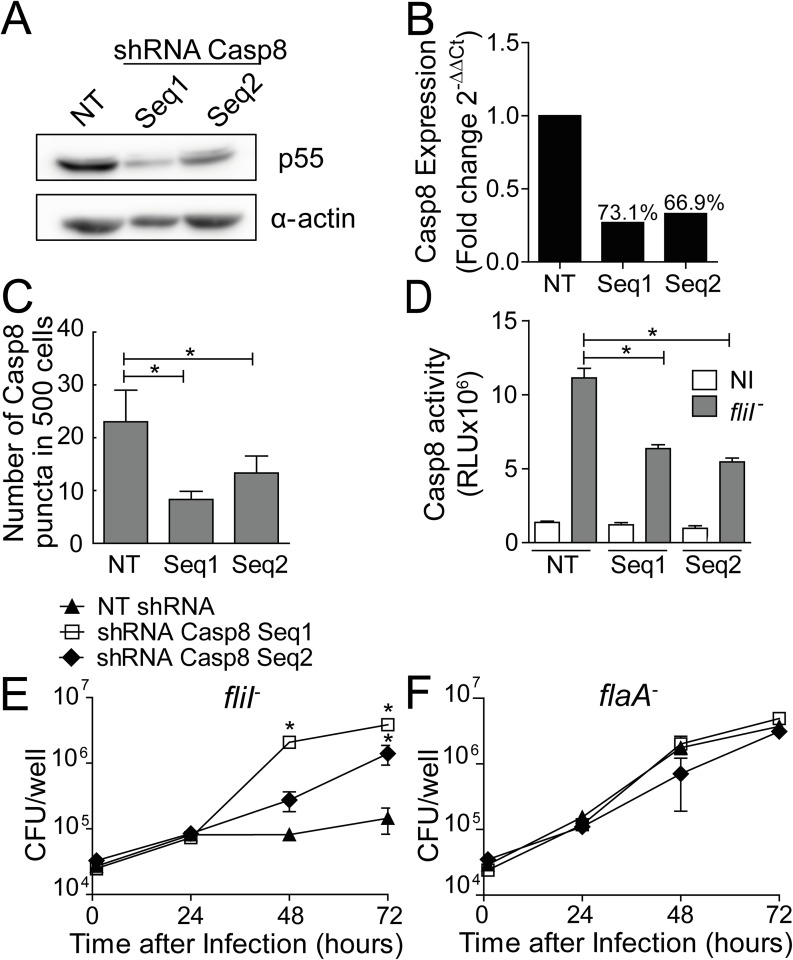
Fig 4. Caspase-8 is important for NLRC4-mediated restriction of L. pneumophila replication independently of caspase-1/11.
Bone marrow-derived macrophages (BMDMs) generated from Casp1/11-/- mice were transduced with a retrovirus encoding shRNA sequences to target caspase-8 (Seq1, Seq2) and a non-target shRNA sequence (NT). (A) The silencing was confirmed by western blot analysis and Real Time qPCR. (A) Cell lysates were separated by SDS-PAGE, blotted and probed with anti-caspase-8 (pro-caspase-8 p55) and anti-α-actin. (B) Quantification of the casp8 gene expression by Real Time qPCR. Actin beta gene was used as a control for normalization of expression levels. The number above the bars indicates the percentage of silencing compared to the NT sequence. (C-D) The transduced Casp1/11-/- BMDMs were infected with motility-deficient L. pneumophila mutants expressing flagellin (fliI-) at a MOI of 10. After 8 hours the cells were fixed, the number of caspase-8 puncta was quantified using an epifluorescence microscope (C) and the activity of caspase-8 was measured using the Caspase-8 Glo Assay (D). (E-F) The transduced Casp1/11-/- BMDMs were infected with fliI- (E) and flaA- (F) at a MOI of 10 to evaluate bacterial replication. The cells were incubated for 24, 48 and 72 hours for CFU determination. Data show the average ± SD of triplicate wells. *P<0.05, compared with NT. (C-D) Student’s t test, (E-F) ANOVA. RLU, relative luminescence units; NI, uninfected. Data are presented for one representative experiment of three experiments with similar results.