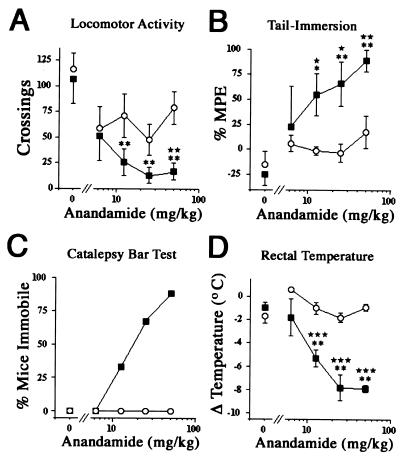
Figure 2.
Pharmacological activity of anandamide in FAAH+/+ and FAAH−/− mice. Anandamide elicited dose-dependent pharmacological effects in FAAH−/− mice (■), but failed to produce any significant effects in FAAH+/+ mice (○). (A) Hypomotility (locomotor activity), ED50 < 6.25 mg/kg; (B) antinociception (tail-immersion), ED50 [95% confidence limits (CL)] = 13 (5–30) mg/kg; (C) catalepsy, ED50 (95% CL) = 20 (11–35) mg/kg; (D) hypothermia (rectal temperature), ED50 (95% CL) = 11 (6–19) mg/kg. ★, P < 0.05; ★★, P < 0.01; and ★★★, P < 0.001, for FAAH−/− versus FAAH+/+ mice receiving the same treatment (planned comparison). *, P < 0.05 and **, P < 0.01 for anandamide-treated versus vehicle-treated FAAH−/− mice (Dunnett's test). The results are presented as means ± SE. n = 6–8 mice per group.