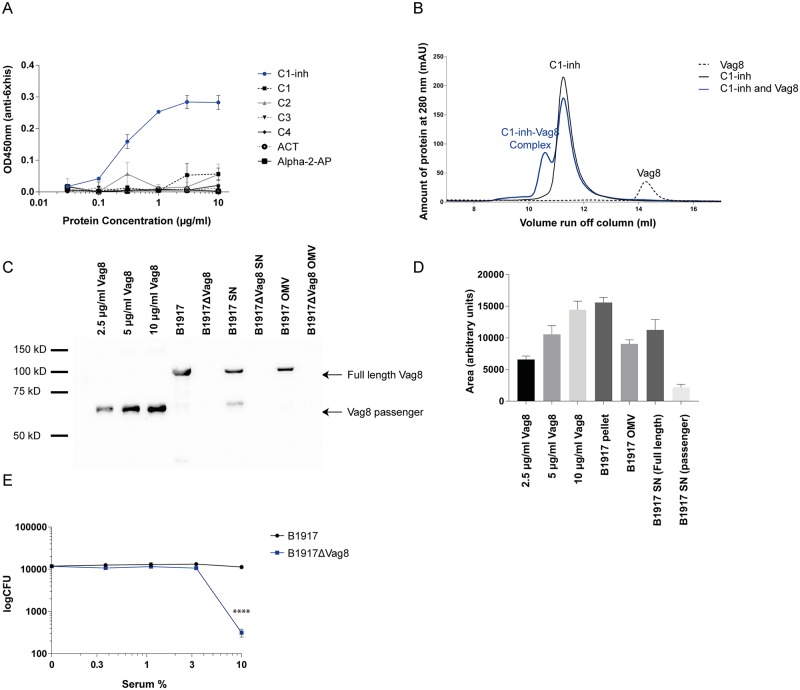
Fig 1. Vag8 binds C1-inh and is involved in serum resistance.
(A) Vag8 binds to C1-inh in a dose dependent manner. No binding was observed to the serpins alpha-1-antichymotrypsin and alpha-2-antiplasmin or the complement components C1, C2, C3 and C4 by ELISA. (B) Vag8 forms a stable complex with C1-inh in fluid phase as shown by making use of the gel filtration chromatography method. (C) The successful construction of the B. pertussis B1917ΔVag8 mutant strain was confirmed by immunoblot. No Vag8 could be detected in the pellet or the bacterial supernatant or OMV’s of B1917ΔVag8. In addition, the B. pertussis wild type strain B1917 expressed both the full length Vag8 as well as the passenger domain in the supernatant. (D) Using ImageJ, the intensity of the Vag8 bands were semi quantified relative to known concentrations of recombinant Vag8. We show that 107 bacteria of the B1917 parental strain contain 10 μg/ml Vag8 and 109 bacteria secrete 5–10 μg/ml of full length and 1 μg/ml of passenger Vag8. Moreover, 10 μg/ml OMV contains 5 μg/ml of Vag8. (E) The B1917ΔVag8 mutant strain shows increased sensitivity to serum-mediated killing compared to the B1917 parent strain. Data shown in Fig 1A, 1D and 1E represent the mean ± SEM of three separate experiments while Fig 1B and 1C are representative of three separate experiments.