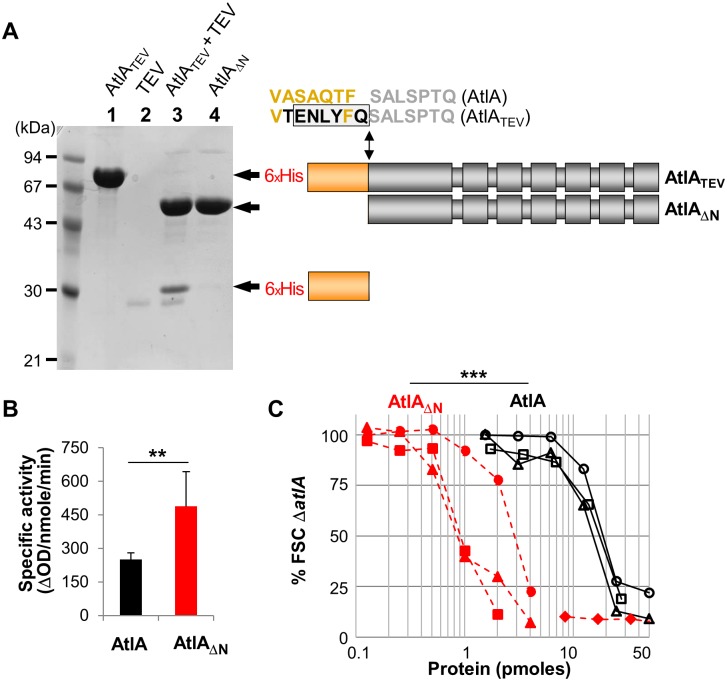
Fig 2. Truncation of AtlA N-terminal domain enhances septum cleavage activity in vitro.
A. SDS-PAGE analysis of purified recombinant proteins: lane 1, full-length AtlATEV (residues 53–737) corresponding to the mature protein (without the signal peptide), with a TEV site between domains 1 and 2; lane 2, TEV protease (TEV); lane 3, TEV digestion product of AtlATEV (AtlATEV+TEV); lane 4, AtlA truncated of its N-terminal domain (AtlAΔN). The amino acid sequences between N-terminal (orange) and catalytic (grey) domains in AtlA and AtlATEV are described. The TEV cleavage site is boxed. B. Comparison of specific enzymatic activities of AtlA and AtlAΔN in vitro using whole PG sacculi as a substrate; **P = 0.0018; n = 9. C. Flow cytometry analysis of septum cleavage activity of recombinant full-length AtlA and the N-terminally truncated variant (AtlAΔN). Activity is expressed as a percentage of the median forward scattered (FSC) light value corresponding to cell chains formed by the atlA mutant (ΔatlA) used as a substrate; ***P = 0.0008; n = 3.