Figure 1.
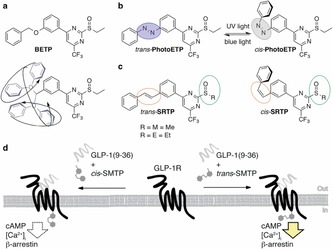
Logic and design of prearranged GLP‐1R positive allosteric modulators. a) BETP possesses an O‐benzyl ether that can freely rotate and adopt various conformations, occupying a large space, as denoted in the lower drawing. b) PhotoETP 3 endows GLP‐1R with light sensitivity when the azobenzene is locked in its active trans‐state under blue light illumination (blue circle), which is interchangeable with UV light to its inactive cis‐state (gray circle). c) Novel stilbene congeners are constantly locked (orange circle) in their respective state and do not exhibit photostationary states. The functional group for displacement can be a methyl (Me) or ethyl (Et) sulfoxide (green circle), giving pure trans‐ and cis‐isomers of the prearranged PAMs SMTP and SETP. d) Schematic representation of GLP‐1R activation with GLP‐1(9–36)NH2 in the presence of either trans‐ or cis‐SMTP.
