Figure 4.
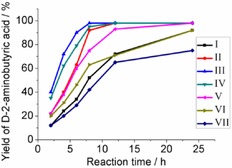
Time course for the formation of d‐2‐aminobutyric acid with different amounts of enzymes and varied concentrations of substrates. The reactions were conducted in Na2CO3–NaHCO3 buffer (100 mm, pH 9.0), and monitored at different time intervals. I: 0.7 U EcTAL, 1 U M‐StDAPDH, 2 U PFDH, 100 mm l‐threonine, 150 mm ammonium formate; II: 2 U EcTAL, 1 U M‐StDAPDH, 2 U PFDH, 100 mm l‐threonine, 150 mm ammonium formate; III: 2 U EcTAL, 2 U M‐StDAPDH, 4 U PFDH, 100 mm l‐threonine, 150 mm ammonium formate; IV: 2 U EcTAL, 2 U M‐StDAPDH, 4 U PFDH, 200 mm l‐threonine, 300 mm ammonium formate; V: 2 U EcTAL, 2 U M‐StDAPDH, 4 U PFDH, 200 mm l‐threonine, 300 mm sodium formate; VI: 2 U EcTAL, 2 U M‐StDAPDH, 4 U PFDH, 300 mm l‐threonine, 450 mm ammonium formate; VII: 2 U EcTAL, 2 U M‐StDAPDH, 4 U PFDH, 300 mm l‐threonine, 450 mm sodium formate.
