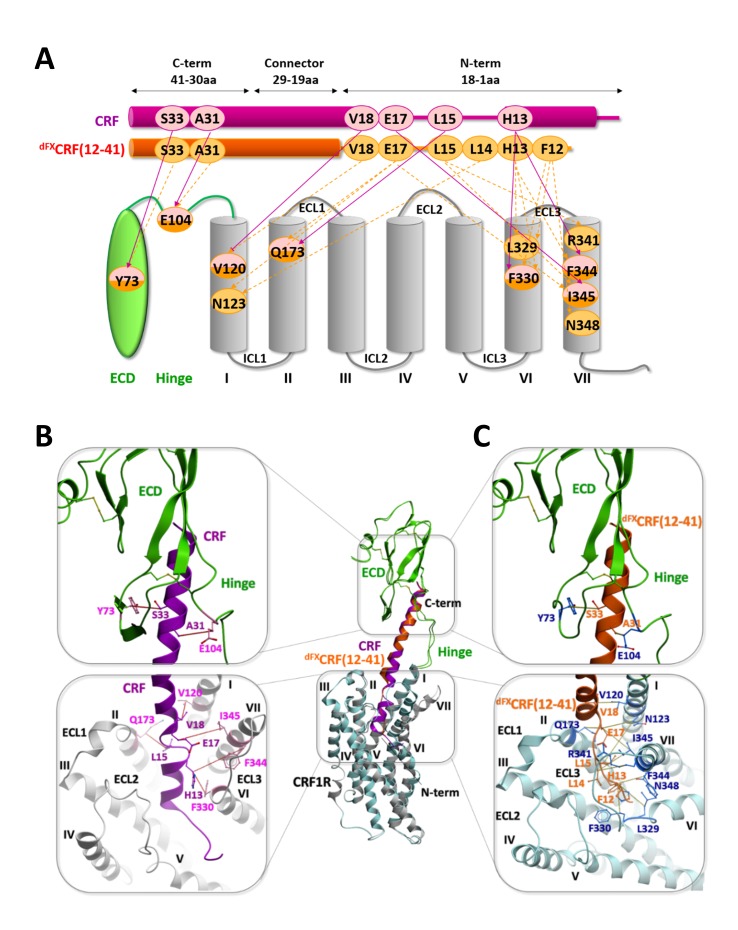
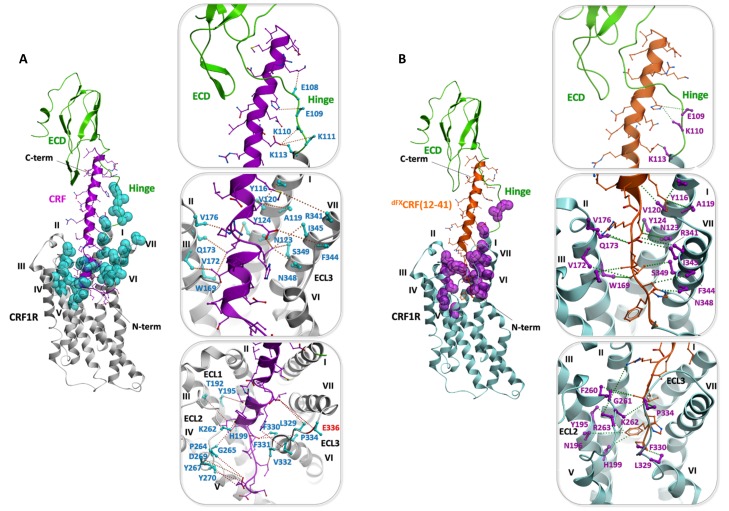
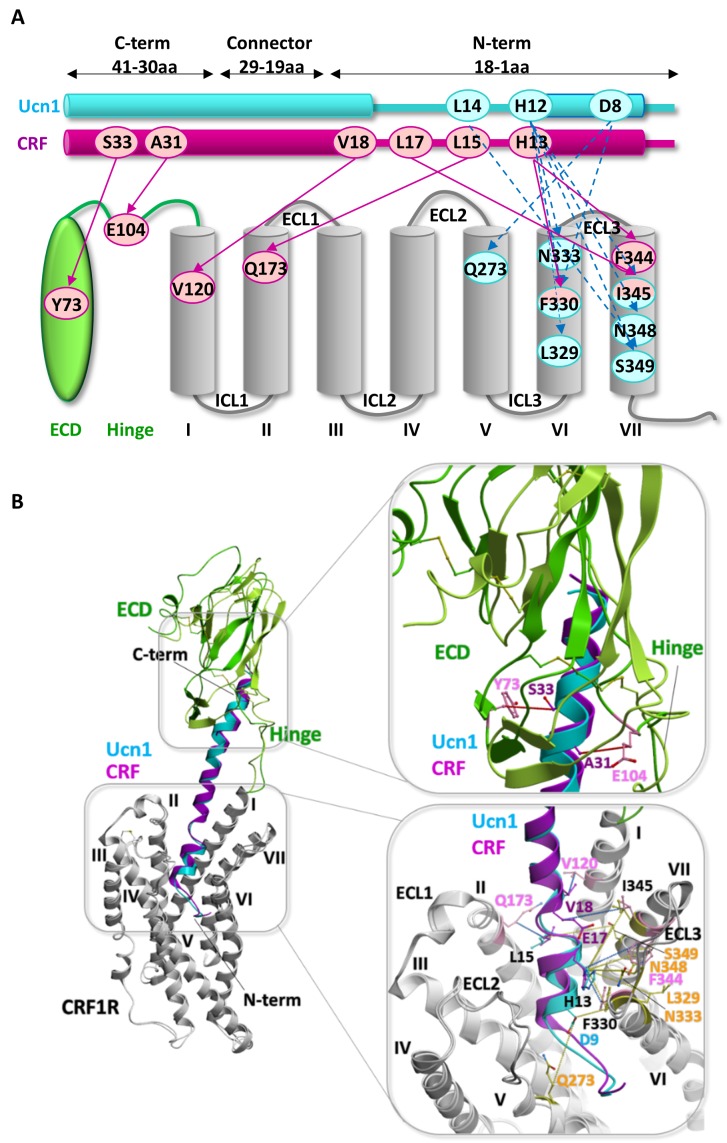
(
A) Pair-wise crosslinking hits of Ucn1 (cyan) and CRF (magenta) with CRF1R. Notably, pair-wise crosslinking was performed using different electrophilic moieties and a different experimental approach in the two cases. For the Ucn1-CRF1R complex, the electrophilic p-2′-fluoroacetylphenylalanine (Ffact) was genetically incorporated into the receptor and Cys residues were incorporated into the ligand. For the CRF-CRF1R complex, the Lys(ClAc) electrophilic moiety was incorporated into the ligand and Cys in the receptor. The two different approaches yielded two distinct sets of pair-wise crosslinking hits, sharing only one overlapping restraint to F330
6.56 (H13
CRF, H13
Ucn1). The models of both peptide complexes, however, are absolutely consistent with each other, validating the predictive power of the models. (
B) Side view of the superimposed models of the Ucn1 (cyan)-CRF1R complex and the CRF (magenta)-CRF1R complex. The peptide-binding pocket is magnified showing the interactions with ECD and TMD. Residue pairs shown in sticks (Ucn1: cyan and yellow, CRF: magenta and pink) and connected by dotted lines (Ucn1: blue, CRF: red, distances are presented in
Table 3) indicate distance restraints derived from pair-wise crosslinking.