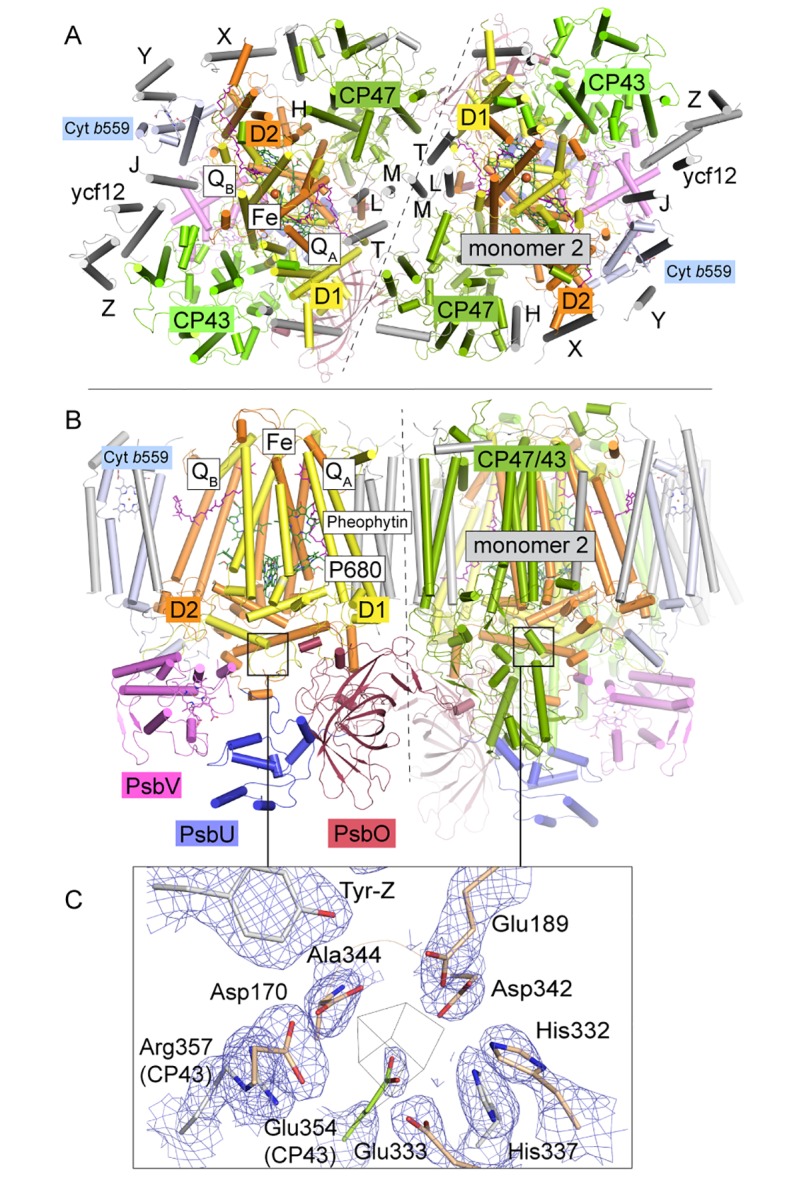
Figure 1. T. elongatus PSIIcc dimers viewed.
(A) from the cytoplasmic side and (B) from the membrane. The reaction centre transmembrane subunits D1/D2, the internal antennae (CP43/CP47, omitted in the left monomer) and the membrane extrinsic subunits PsbU/V/O are highlighted in color. Small transmembrane subunits comprising a single α-helix (two for PsbZ) are indicated by single letter referring to the PsbH-Y proteins and ycf12. QA/B denote the plastoquinone cofactor, Fe the non-heme iron. The site of the water-oxidizing complex (WOC, Mn4CaO5) within each monomer is shown by the black box. (C) Electron density map (1σ 2Fo-Fc) at the depleted WOC site (shown for the locked dimer, discussed below). Free coordinating residues from the D1 subunit (cream) and CP43 (green) are shown alongside Arg357(CP43) and His 337(D1), which are within hydrogen-bond distance to cluster oxygen atoms in Umena et al. (2011), (PDB:3WU2).