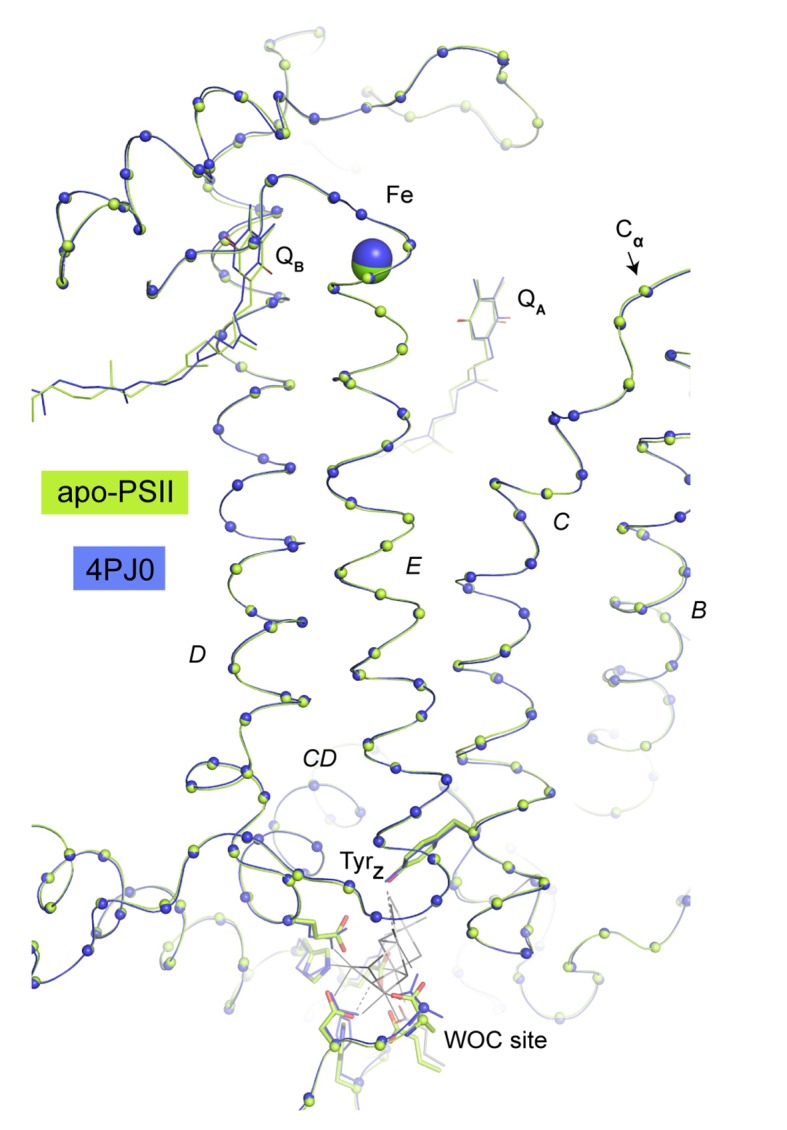
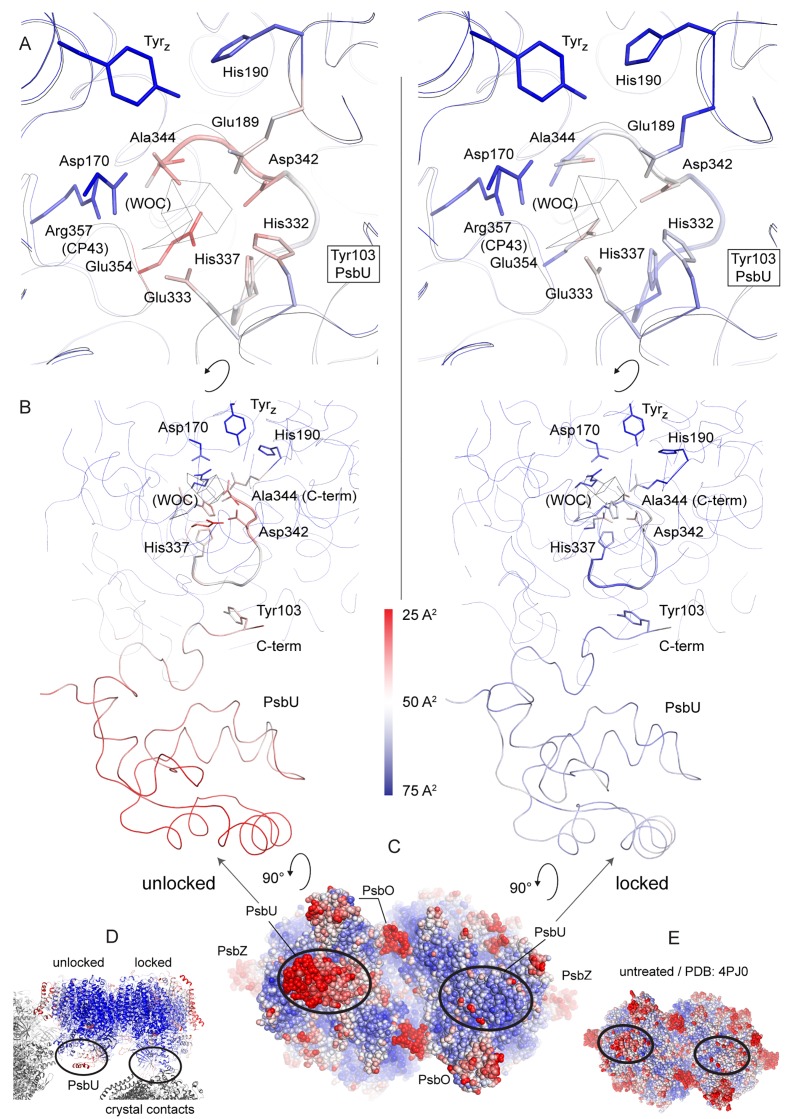
Figure 3. Comparison between the locked and unlocked monomer.
(A) Locked (right) and flexible, unlocked monomer (left). Atomic B-factors are shown using the indicated color coding. The inset shows the crystal contacts acting on PsbU in the right monomer. (B) A slice through the unlocked PSII monomer showing D1 and extrinsic, manganese stabilizing subunits PsbU, PsbV, PsbO (the 147–193 loop and the position of the external β-barrel). The inset shows the WOC-binding site and C-terminal loops of both PsbU and D1 including, residues involved in their interaction (Tyr103 and Pro340) and residues involved in cluster binding in intact PSII.
Figure 3—figure supplement 1. Overlay of Cα atoms (spheres) of D1 residues between the WOC site and the acceptor site (Fe, QA, QB).