Figure 5.
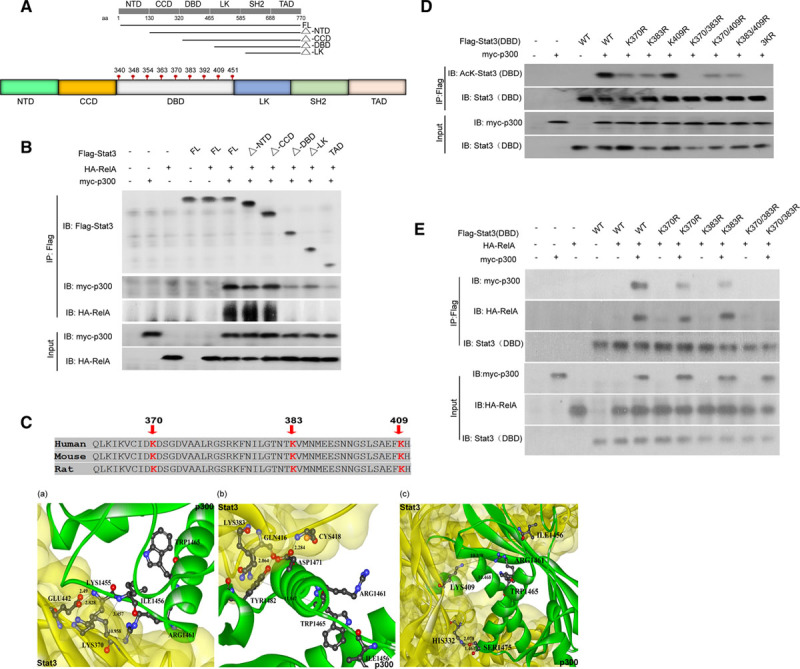
Acetylation of Stat3 at LYS(K)370, LYS(K)383, or both enhanced its interactions with RelA. A, Schematic diagram for various truncated mutant of Stat3 that span different domains of Stat3 as denoted by straight black line. The truncated mutant Stat3 lacking N-terminal domain (NTD) is denoted by ΔNTD. The same scheme was used for the other truncated mutants, which are noted by Δ coiled-coil domain (CCD), Δ DNA-binding domain (DBD), and Δ linker domain (LK). Different amino acid (aa) sites were also marked for each domain. All the lysine residues located within the DBD, which is labeled in gray, are outlined. B, HEK293T (human embryonic kidney 293T) cells were transfected with HA-RelA or myc-p300, as well as different vectors containing various Flag-tagged Stat3 mutants (as indicated in A). The Flag-tagged Stat3 mutants were immunoprecipitated and then immunoblotted using specific antibodies against Flag, myc, and HA. C, The Stat3–p300 interface was simulated by an in silico analysis focusing on the lysine residues K370 (a), K383 (b), and K409 (c), which served as binding sites. The distance between the residues on Stat3 and p300 is shown in the figure. D, Various vectors containing a Stat3 DBD in which the putative lysine residues found in Flag-Stat3(DBD), namely, LYS(K)409, K383, and K370, were mutated to arginine (R) either alone or in combination, as indicated, were generated and transfected into HEK293T cells along with myc-p300. Whole-cell proteins were then harvested for immunoprecipitation, which was performed using antibodies against Flag-tag, followed by immunoblotting using antibodies against acetyl-lysine to assess the acetylation of the mutant Stat3 DBD. E, The same HEK293T cells described in D were also transfected with HA-RelA before undergoing the same tests to determine whether p300-mediated Stat3 acetylation also affects Stat3/RelA interactions. ARG indicates arginine; ASP, aspartic acid; CYS, cysteine; FL, full-length Stat3; GLN, glutamine; GLU glutamic acid; HIS, histidine; ILE, isoleucine; SER, serine; SH2, Src homology 2 domain; TAD, that only the transactivation domain was present in the corresponding truncated mutant; TRP, tryptophan; and TYR, tyrosine.
