Fig. 7.
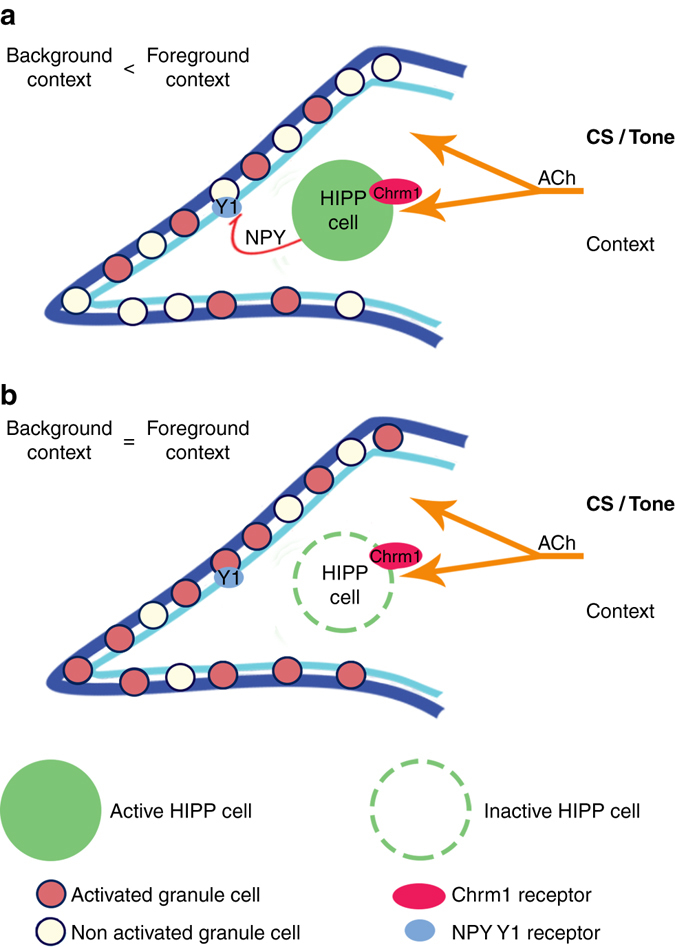
A local circuit model for the cholinergic modulation of background context salience in the dentate gyrus. a HIPP cells act as relay stations in the septo-hippocampal pathway that adjust context memory strength during fear learning. Acetylcholine released from medial septal afferences during memory acquisition activates both granule cells and HIPP cells in the dentate gyrus. The cholinergic stimulation of HIPP cells via muscarinic M1 receptors (Chrm1) triggers their NPY release, which in turn via Y1 receptors attenuates the excitation of granule cells. This feed forward inhibition is effective when conditioning occurs to an elemental cue (context in background) and restricts the number of granule cells that are recruited to the fear memory engram, resulting in reduced context salience. b Upon experimental silencing of HIPP cells and blockage of NPY-mediated inhibition the cue-induced devaluation of the background context fails and an increased number of granule cells are recruited to the context memory trace. Experience-dependent changes in HIPP cell function, e.g., via CREB-mediated transcription can adjust the strength of this local circuit and are critical for the function of the DG in adaptively encoding context salience
