Figure 2.
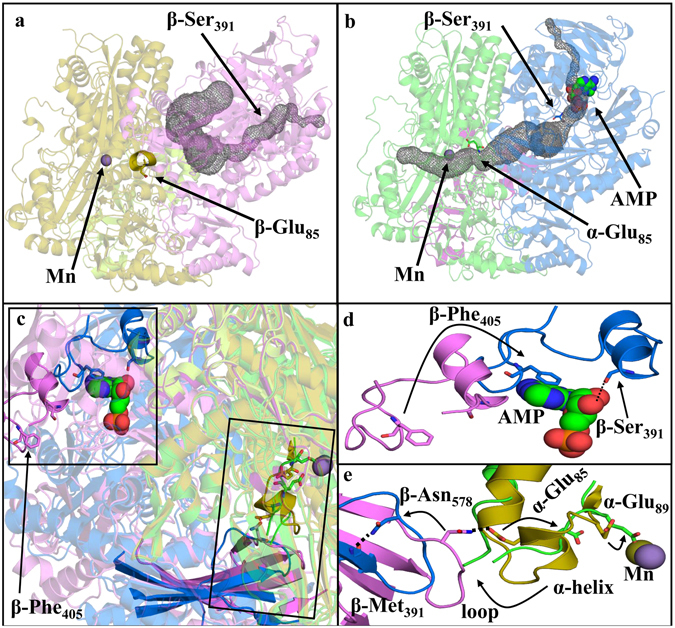
Conformational shift upon nucleotide binding. (a) Ligand-free structure (α subunits: olive; β subunits: violet; γ subunits: limon) showing a substrate channel (grey) linking the nucleotide binding site to solvent and allowing ATP and substrates to enter. Access to the Mn site is closed off in this structure by an Mn proximal α-helix that prevents the substrate channel from reaching the Mn active site. (b) AMP-bound structure (α subunits: green, β subunits: blue, γ subunits: pink) showing an opening of an internal channel (grey) linking the nucleotide binding site to the Mn binding site. The Mn proximal helix becomes a disordered loop region when AMP is bound, permitting access to the Mn. (c) Superposition of the ligand-free structure on the AMP-bound structure. The structures as a whole are rendered semi-transparently. Boxes highlight regions with important changes, and key features are rendered in fully-opaque colors. (d) Close up of the upper boxed region in (c) illustrating pronounced changes in the positions of β-Phe405 and β-Ser391 upon binding of nucleotide. (e) Detailed representation of the lower boxed region in (c). A cross-subunit β-Asn578/α-Glu85 interaction in the ligand-free structure is disrupted following AMP binding. This allows α-Glu85 to move in the direction of the arrow and leads to destabilization of the Mn-proximal α-helix shown in (a). These changes together allow the α-Glu89 side chain to rotate to coordinate to the Mn.
