Figure 5.
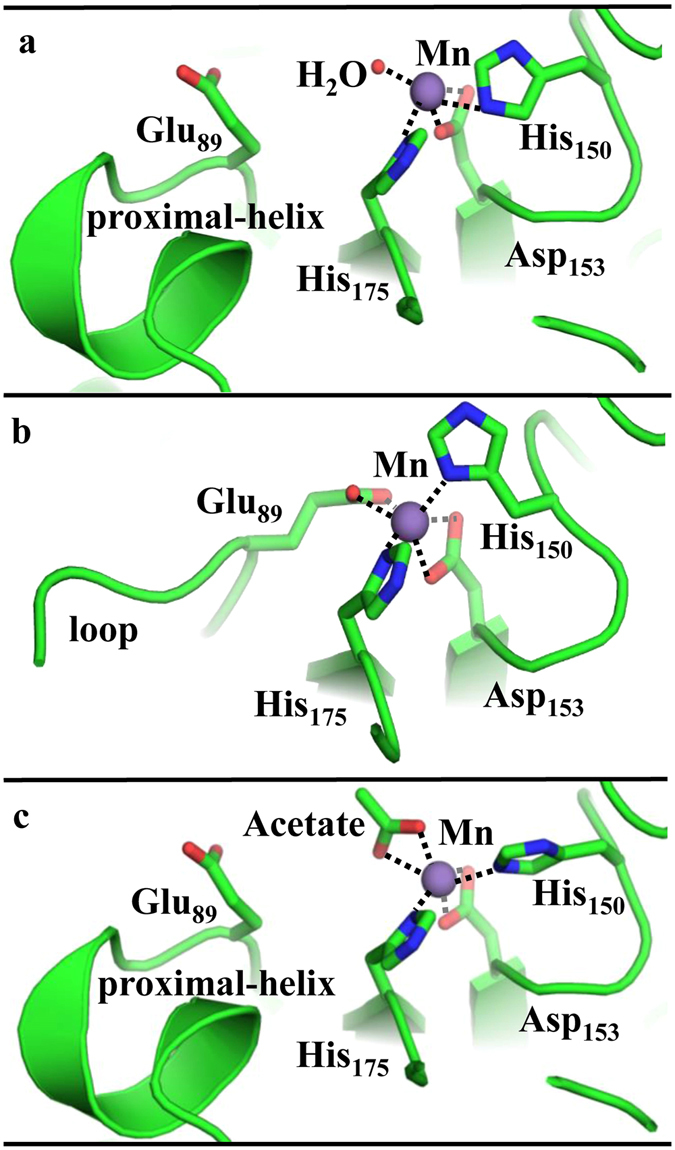
Conformational changes enforce changes in coordinating residues in the Mn active site. (a) The ligand-free structure shows Mn coordinated to a water, His150, His175, and Asp153 (all deriving from the α-subunit). The Mn-proximal α-helix is structured, blocking the Mn site from access to the α/β interface and solvent. (b) The AMP-bound structure shows Mn coordinated to His150, His175, Asp153, and Glu89. Nucleotide binding to the β subunit induces large conformational changes that cause the Mn-proximal α-helix to form a disordered loop, allowing the Glu89 side chain to coordinate to the Mn site. (c) Acetate-bound structure shows the displacement of Glu89 and the concomitant reorganization of the Mn-proximal α-helix. The internal channel is closed and the substrate-access channel is open in this structure, which is proposed to illustrate the effects of displacement of Glu89 from the Mn by phosphorylated intermediates.
