Figure 6.
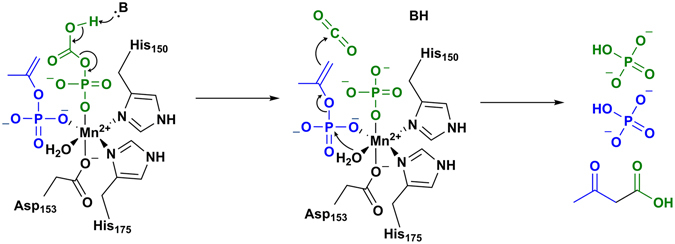
Reaction of intermediates to form products. A Mn active site mechanism of action is proposed. At the Mn active site, we propose a mechanism of action in two steps. It is reasonable to believe from our acetate-bound structure that the Mn is responsible for the ordering of the intermediates. (A) First, a residue near the carboxyl side of the carboxyphosphate will deprotonate the carboxylic acid which triggers a decarboxylation event to produce CO2 and an inorganic phosphate. This initial reaction contains similarities to the PEPC Mn active site where a histidine residue stabilizes the carboxyphosphate intermediate, the AC-acetate bound structure shows His111 in proximity to the active site and can serve an analogous role. (B) After the decarboxylation event, the enol-acetone will then bond with the carbon dioxide after hydrolysis of its phosphate group. (C) The products of the reaction acetoacetate and two inorganic phosphates will then exit through the dimer interface restarting the Mn active site.
