Figure 5.
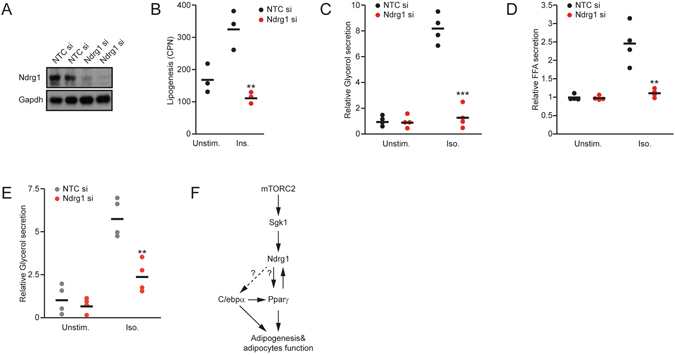
Ndrg1 promotes lipolysis independent of its impact on adipocyte differentiation. (A) Western blot for the indicated proteins in differentiated 3t3l1 cells transfected with control or Ndrg1 siRNA. (B) Lipogenesis rate in control siRNA and Ndrg1 siRNA transfected cells (Ndrg1 si), unstimulated (unstim.) or upon insulin (Ins.) stimulation. (C) Relative glycerol and (D) FFA levels in medium from differentiated 3t3l1 control cells or Ndrg1 siRNA-treated cells stimulated with control medium or Iso. (E) Relative glycerol levels in adipocytes differentiated from primary stroma-vascular cells isolated from subcutaneous fat pads and transfected with non-targeted control (NTC) or Ndrg1 siRNA. Each data point represents a biological replicate. Stars indicate significance for given parameters between control and Ndrg1-depleted cells. (*P < 0.05, **P < 0.01, ***P < 0.001 according to ANOVA followed by the Post hoc Tukey test). (F) Model of Ndrg1 action in adipocytes. mTORC2 activates Sgk1 kinase, which phosphorylates Ndrg1 on T346. Phosphorylated Ndrg1 increases expression of Pparγ through an unknown mechanism to promote adipogenesis and adipocyte function. In a positive feedback loop, Pparγ also promotes Ndrg1 expression and stability. Additionally, Ndrg1 promotes C/Ebpα phosphorylation, which might influence its activity.
