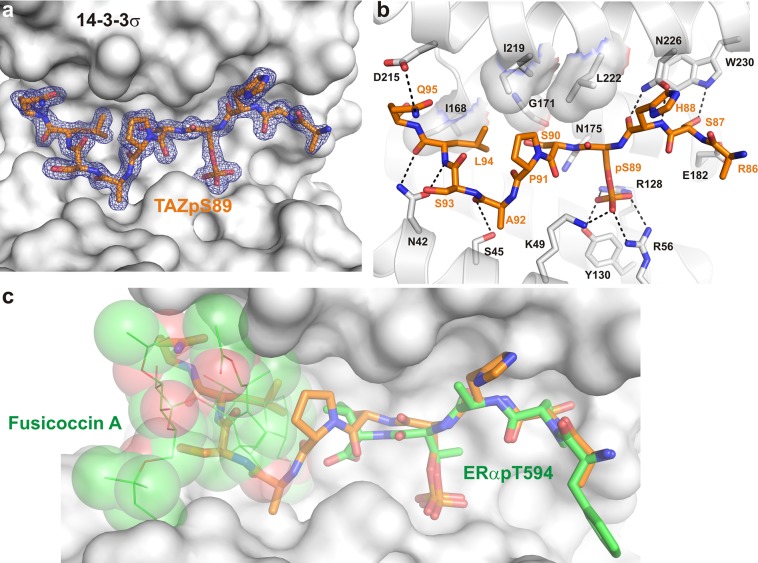
Figure 1.
Crystal structure of the 14-3-3σ/TAZpS89 complex. (a) Final 2Fo – Fc electron density (blue mesh, contoured at 1.0σ) of the TAZpS89 peptide (orange sticks) bound to 14-3-3σ (white surface). (b) Details of the 14-3-3σ/TAZpS89 interaction. Residues of 14-3-3σ that are important for binding of TAZpS89 are shown as white sticks; polar interactions are depicted as black dotted lines, and hydrophobic contacts are displayed as a semitransparent surface. (c) Surface representation of the 14-3-3σ/TAZpS89 interface in overlay with ERα (green sticks) and Fusicoccin A (green lines and semitransparent spheres). The small molecule binding site is unavailable in the 14-3-3σ/TAZpS89 structure as the central binding channel of 14-3-3 is almost entirely filled by the TAZ peptide.