Figure 1.
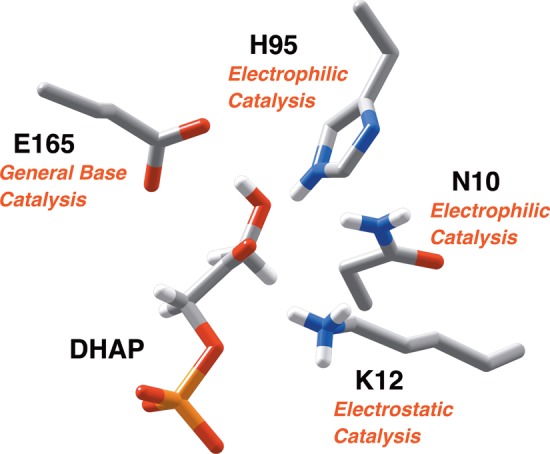
A representation of the catalytic side chains at the active site of TIM (PDB ID: 1NEY), using the numbering of the residues from the wild-type TIM from Saccharomyces cerevisiae.15,19,20 The substrate dihydroxyacetone phosphate (DHAP) is deprotonated by the carboxylate side chain of E165.21,22 The neutral imidazole side chain of H95 is positioned to stabilize negative charge that develops at O-1 or O-2 of the isomeric enediolate phosphate reaction intermediates,23,24 and the amide side chain of N10 is positioned to stabilize negative charge that develops at O-1.25 The alkyl ammonium cation side chain of K12 interacts with both the phosphodianion and the negatively charged oxygen of an enediolate phosphate reaction intermediate.26−29
