Figure 2.
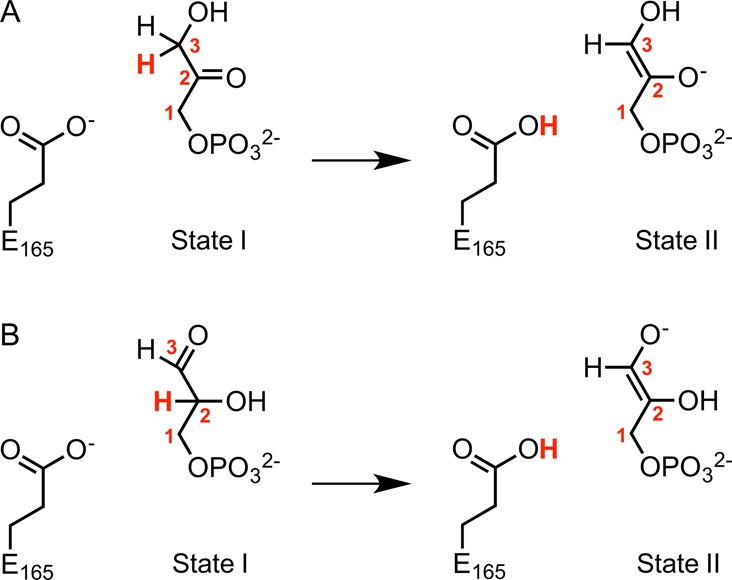
Valence bond states used in this work to describe the TIM-catalyzed deprotonation of the enzyme-bound substrates (A) DHAP and (B) GAP. State I and State II correspond to complexes to the reactant and to the enediolate phosphate intermediate, respectively. In the case of the corresponding uncatalyzed reaction, the carboxylate side chain of E165 was modeled using a propionate anion. The numbering of the carbon atoms of the two substrates is shown, and the transferred proton is highlighted in bold red text.
