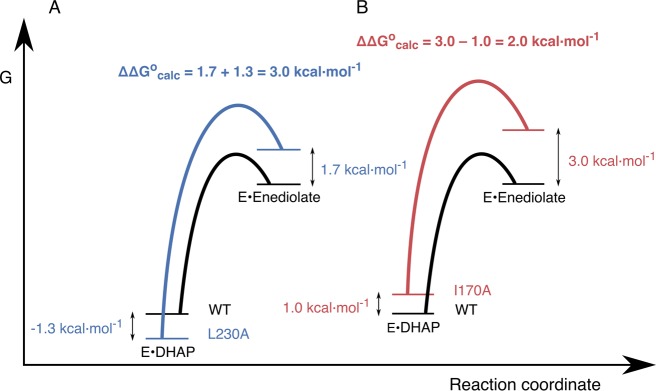
Figure 9.
Free energy profiles for wild-type and mutant TIM-catalyzed deprotonation of enzyme-bound DHAP, where the energy of the Michaelis complex is shown relative to the energy for unliganded TIM and DHAP. (A) Profiles for the reactions catalyzed by wild-type TIM and the L230A mutant. (B) Profiles for the reactions catalyzed by wild-type TIM and the I170A mutant. The values of ΔΔGR°, ΔΔGI, and ΔΔG° = ΔG°mut – ΔG°WT from Table 2 are −1.3, 1.7, and 3.0 kcal mol–1 for the L230A mutant, and 1.0, 3.0, and 2.0 kcal mol–1 for the I170A mutants, respectively.