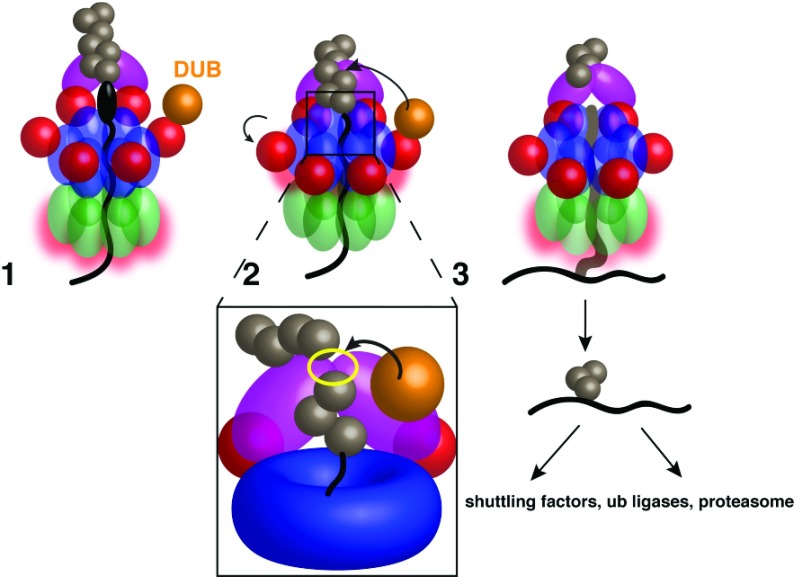
Figure 3. Model for substrate release from Cdc48.
Stage 1: The substrate is pulled through the double-ring ATPase, but the polyubiquitin chain is bound to the Ufd1/Npl4 (UN) complex at the cis side, preventing full substrate translocation through the pore, even with continued D2 ATP hydrolysis. A deubiquitinase (DUB) bound to the N domain cannot access the polyubiquitin chain while D1 is in the ATP-bound state and the N domains are in the “up conformation”. Stage 2: D1 hydrolyzes ATP after the substrate has initiated or completed translocation, moving the N domains into the “down conformation” and allowing DUB access to the ubiquitin chain. Inset: The likely Ub chain cleavage site is between the UN binding site (yellow outline) and the central pore; any Ub moieties proximal to the cleavage site will be translocated. Stage 3: After polyubiquitin cleavage, the oligoubiquitinated substrate proceeds fully through the pore. The ubiquitin moieties probably refold after translocation, and the substrate is transferred to downstream factors.