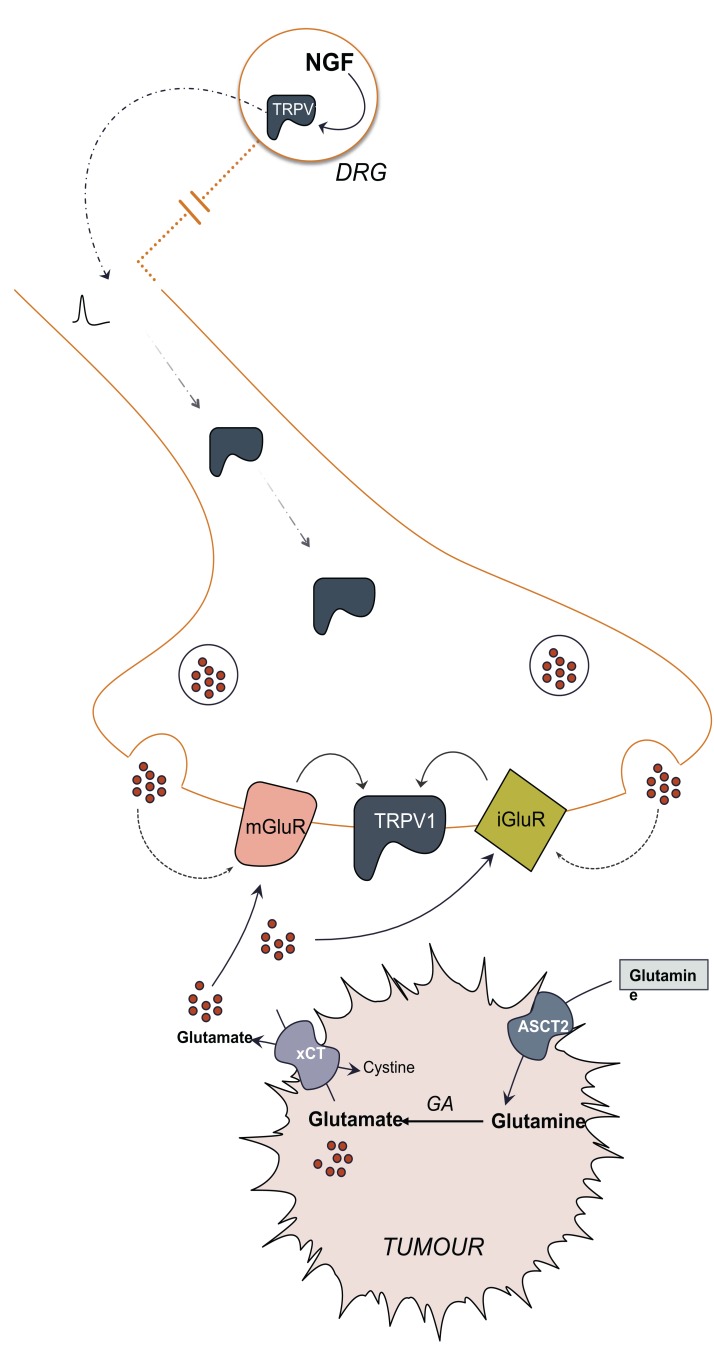
Fig. (3).
Overview of peripheral nociception induced by tumour-derived glutamate. Dysregulated cancer cell metabolism promotes glutamine uptake by ASCT2 transporter and production of large intracellular glutamate pools that drive the activity of the cystine/glutamate transporter, xCT to accommodate the intracellular demand for cysteine, the limiting reagent in glutathione synthesis. Upregulation of glutaminase (GA) and system xc- increases the extracellular concentration of glutamate that can be perceived by proximal nociceptive terminals that can translate glutamate into a nociceptive signal by integrating the activities of glutamate receptors with TRPV1. These terminals also release glutamate that can act in an autocrine fashion also activating these same glutamate receptors. TRPV1 translocation to these terminals increases in response to peripheral noxious stimuli through the action of NGF in the dorsal root ganglion.