Abstract
Background
Evidence-based recommendations for treating persons having presumed latent tuberculosis (LTBI) after contact to infectious multidrug-resistant (MDR) tuberculosis (TB) are lacking because published data consist of small observational studies. Tuberculosis incidence in persons treated for latent MDR -TB infection is unknown.
Methods
We conducted a systematic review of studies published 1 January 1994–31 December 2014 to analyze TB incidence, treatment completion and discontinuation, and cost-effectiveness. We considered contacts with LTBI effectively treated if they were on ≥1 medication to which their MDR-TB strain was likely susceptible. We selected studies that compared treatment vs nontreatment outcomes and performed a meta-analysis to estimate the relative risk of TB incidence and its 95% confidence interval.
Results
We abstracted data from 21 articles that met inclusion criteria. Six articles presented outcomes for contacts who were treated compared with those not treated for MDR-LTBI; 10 presented outcomes only for treated contacts, and 5 presented outcomes only for untreated contacts. The estimated MDR-TB incidence reduction was 90% (9%–99%) using data from 5 comparison studies. We also found high treatment discontinuation rates due to adverse effects in persons taking pyrazinamide-containing regimens. Cost-effectiveness was greatest using a fluoroquinolone/ethambutol combination regimen.
Conclusions
Few studies met inclusion criteria, therefore results should be cautiously interpreted. We found a reduced risk of TB incidence with treatment for MDR-LTBI, suggesting effectiveness in prevention of progression to MDR-TB, and confirmed cost-effectiveness. However, we found that pyrazinamide-containing MDR-LTBI regimens often resulted in treatment discontinuation due to adverse effects.
Keywords: tuberculosis, multidrug-resistant, treatment, contacts, cost
Evidence-based recommendations for treatment of persons (ie, contacts) having presumed multidrug-resistant latent tuberculosis (MDR-LTBI) after contact to a patient with infectious MDR tuberculosis (TB) are lacking because published data consist of small observational studies. Three international clinical trials of MDR-LTBI regimens are ongoing in household contacts, with expected completion in 2021: (1) Tuberculosis child and adolescent multidrug-resistant preventive therapy trial (TB CHAMP) (levofloxacin [LEV] vs placebo in children/adolescents); (2) V-QUIN (LEV vs placebo in children/adolescents); and (3) protecting households on exposure to newly diagnosed index multidrug-resistant tuberculosis patients (PHOENIx) (delamanid vs isoniazid) [1]. Current guidance for managing persons having contact with infectious MDR-TB is therefore based on expert opinion.
The US Centers for Disease Control and Prevention (CDC) published guidance in 1992 and 2000 that advised (1) no treatment for MDR-LTBI for persons not at high risk for progression to TB, but provision of clinical follow-up for TB signs and symptoms, and (2) 6–12 months of treatment with ≥2 medications to which the isolate of the source case is susceptible [2]. The Curry International Tuberculosis Center suggests an MDR-LTBI regimen of levofloxacin or moxifloxacin alone or combined with a second medication to which the isolate of the infectious source patient is susceptible [3]. A recent policy brief published by Harvard Medical School after review of existing evidence stated that “further evidence is urgently needed in this field and findings from the planned clinical trials are keenly awaited. However, in the interim, action can be taken. Postexposure management of household contacts of MDR-TB is effective, feasible, and cost efficient, and could be implemented immediately” [4].
There have been recently published studies of the use of treatment for MDR-LTBI that add to existing literature and might provide additional evidence to address the gaps in knowledge that influence MDR-LTBI treatment guidelines. TB incidence in persons having contact with infectious MDR-TB who were treated for MDR-LTBI has not been aggregated through meta-analysis and treatment discontinuation due to adverse effects has not been systematically documented.
Our goal was to assess, using systematic review and meta-analysis, whether MDR-LTBI treatment is significantly associated with lower TB incidence, compared with no medical treatment. We also examined treatment completion, adverse effects, and cost-effectiveness.
METHODS
We developed and examined a population, intervention, comparison, and outcome (PICO) question following Cochrane procedures [5]. Our PICO question was: “Among contacts to infectious MDR-TB patients with presumed MDR-LTBI, should MDR-LTBI treatment compared with no effective medical treatment be used?” We conducted a systematic review of published studies (1 January 1994–31 December 2014) in English or Spanish that presented data on persons having contact to infectious MDR-TB, who had documented LTBI test reactivity or presumed (for children <5 years of age or living with human immunodeficiency virus [HIV]) LTBI, treated or untreated MDR-LTBI, and TB incidence rates. The outcome of MDR-TB incidence was verified by culture and drug susceptibility testing, except for some children, who often are culture negative and thus have no drug susceptibility results. We searched PubMed, Embase, and the Cochrane Library for the keywords tuberculosis, multidrug resistant, contacts, and treatment. We excluded case reports with <10 participants and studies only reporting on the diagnosis or treatment of MDR-TB. We used individual study definitions of contacts and of LTBI, based on positive reactions to tuberculin skin tests (TSTs), but included child contacts <5 years of age or any contacts with HIV as having presumed LTBI and eligible for LTBI treatment regardless of TST result. We considered that persons having contact to infectious MDR-TB were effectively treated for LTBI if they received ≥1 medication (using intention-to-treat categorization) to which their MDR-TB strain was likely susceptible (based on the known/presumed drug susceptibilities of the index patient), otherwise they were not effectively treated. Our definition of effective treatment did not exclude monotherapy despite CDC guidelines, as clinicians have often used monotherapy (usually a fluoroquinolone) for MDR-LTBI treatment, and a mouse model study [6] demonstrated efficacy of fluoroquinolones against latent Mycobacterium tuberculosis. We identified review articles and performed a manual search of their references, but excluded the reviews to avoid duplication. We searched journal article titles and abstracts, then reviewed the full text of selected articles.
We abstracted data on the outcome of TB incidence associated with treatment or no treatment. Person-time TB incidence data were available from few studies and were estimated when unavailable. To analyze TB incidence, we excluded 1 comparison study (Attamna et al [7]) and 1 treatment-only study (Feja et al [8]) that relied solely on registry matches, which are considerably influenced by patient mobility and loss to follow-up, resulting in inability to accurately identify incident TB. Moreover, their inclusion significantly inflates study denominators, creating great meta-analysis heterogeneity. However, we included studies in which registry matches were only 1 component of follow-up to identify incident TB and registry match studies of other outcomes. We selected studies that compared treatment vs nontreatment outcomes and performed meta-analysis to estimate the incident relative risk of TB and its 95% confidence interval (CI). We estimated and controlled for differing person-time of follow-up using Poisson, zero-inflated Poisson, zero-inflated Poisson with random effects, and negative binomial regression methods.
In addition to analyzing data from studies that included persons both treated and untreated for MDR-LTBI in the same study, we aggregated data on TB incidence from studies only reporting on persons treated for MDR-LTBI and separately for studies only reporting on untreated persons.
Where available, we abstracted data on MDR-LTBI treatment completion and adverse effects and report proportions and their CIs. We aggregated individual level adverse effects data by regimen, if they were documented, to compute the proportion of persons on the regimen who experienced adverse effects and the proportion who stopped MDR-LTBI treatment because of adverse effects. We also updated a decision analysis of MDR-LTBI treatment [9] by applying adverse effect rates by regimen.
To assess incremental cost-effectiveness of individual regimens, we applied the regimen-specific completion and adverse effects discontinuation averages from our review, MDR-TB societal costs (excluding deaths) [10, 11] in 2014 US dollars [12], along with efficacy estimates from mouse models [6] and LTBI treatment costs (updated to 2014 dollars) used by Holland et al [9] to conduct a decision analysis (Supplementary Appendix) from the societal perspective of a hypothetical cohort of 100 MDR-TB cases and their contacts per year for 40 years. We also used the following quality-adjusted life year (QALY) estimates: [13] 0.53 alive after MDR-TB, 0.90 alive with MDR-LTBI, 0.80 alive with MDR LTBI post–adverse effect treatment stop, and 0.75 alive with MDR-LTBI posthospitalization for adverse effect treatment stop. We assumed a LTBI reactivation rate of 3% over 40 years, calculated from 2011–2012 National Health and Nutrition Examination Survey data [14]. One-way sensitivity analyses were conducted to assess the effect of increasing adverse effects by 50% with each regimen.
RESULTS
Ninety-five references met inclusion criteria (Figure 1). After reviewing titles and abstracts, we excluded 71 studies, but included additional references from 6 review articles [1, 3, 15–18]. Of 24 remaining studies (22 articles and 2 abstracts), 1 was excluded due to insufficiently reported data. Two conference abstracts were requested from authors, but not received. We reviewed and abstracted full text for 21 articles: 6 “comparison” studies presented outcomes for persons both treated and untreated for MDR-LTBI [7,19–23], 10 presented only outcomes for persons treated [8,24–32], and 5 only outcomes for untreated persons [33–37].
Figure 1.
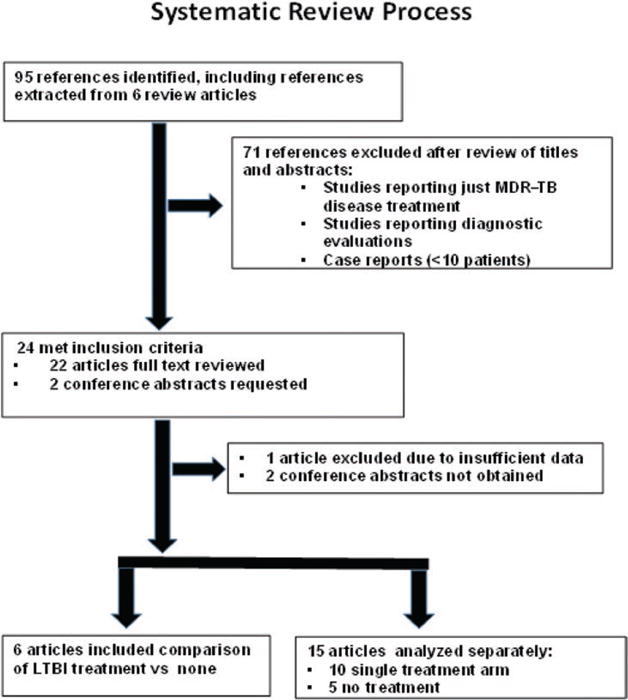
Of 6 comparison studies, 5 (Bamrah et al [19], Denholm et al [20], Schaaf et al [21], Adler-Shohet et al [22], Williams et al [23]) were used for tuberculosis incidence and treatment completion, and 4 (Bamrah, Denholm, Schaaf, Adler-Shohet) for treatment discontinuation due to adverse effects. Of 10 single-treatment-arm studies, 8 (Feja et al [8], Garcia-Prats et al [25], Horn et al [26], Lou et al [27], Miramontes et al [28], Papastavros et al [29], Ridzon et al [30], Seddon et al [31]) contributed to treatment completion and 8 (Feja, Garcia-Prats, Horn, Lou, Papastavros, Ridzon, Seddon, Younossian et al [32]) to treatment discontinuation due to adverse effects. Five no-treatment studies contributed to tuberculosis incidence. Abbreviations: LTBI, latent tuberculosis; MDR, multidrug-resistant; TB, tuberculosis.
Of the 6 comparison studies, all but Bamrah et al [19] (Micronesia) and Schaaf et al [21] (South Africa) were conducted in countries having low TB incidence: Adler-Shohet et al [22] (United States), Attamna et al [7] (Israel), Denholm et al [20] (Australia), and Williams et al [23] (United Kingdom). Among the studies in low-incidence settings, only Denholm et al reported any TB cases (2/49 [4%] study subjects) after 2–6 years following treated and untreated persons. After exclusion of the Attamna et al registry match, 5 comparison studies (Table 1) were included in the meta-analysis.
Table 1.
Tuberculosis Incidence Data from 5 Comparison Studies of Treatment of Presumed Multidrug-Resistant Latent Tuberculosis
| Study | TB | No TB | Total |
|---|---|---|---|
| Adler-Shohet et al [22] | |||
| LTBI Tx | 0 | 26 | 26 |
| No LTBI Tx | 0 | 5 | 5 |
| Total | 0 | 31 | 31 |
|
| |||
| Bamrah et al [19] | |||
| LTBI Tx | 0 | 104 | 104 |
| No LTBI Tx | 3 | 12 | 15 |
| Total | 3 | 116 | 119 |
|
| |||
| Demholm et al [20] | |||
| LTBI Tx | 0 | 11 | 11 |
| No LTBI Tx | 2 | 36 | 38 |
| Total | 2 | 47 | 49 |
|
| |||
| Schaaf et al [21] | |||
| LTBI Tx | 2 | 39 | 41 |
| No LTBI Tx | 13 | 51 | 64 |
| Total | 15 | 90 | 105 |
|
| |||
| Williams et al [23] | |||
| LTBI Tx | 0 | 8 | 8 |
| No LTBI Tx | 0 | 4 | 4 |
| Total | 0 | 12 | 12 |
Overall TB incidence was 3% in Micronesia (Bamrah et al), 4% in Australia (Denholm et al), and 14% in South Africa (Schaaf et al). The individual study relative risks of TB incidence with multidrug-resistant LTBI treatment compared with no treatment, adding 0.5 to zero cells, were: 0.21 (95% confidence interval [CI], .005–9.38) in Adler-Shohet et al; 0.02 (95% CI, .001–.4) in Bamrah et al; 0.83 (95% CI, .04–17.06) in Denholm et al; 0.24 (95% CI, .06–1.01) in Schaaf et al; and 0.53 (95% CI, .01–22.5) in Williams et al.
Abbreviations: LTBI, latent tuberculosis; TB, tuberculosis; Tx, treatment.
Adler-Shohet et al [22] observed 31 children with MDR-LTBI prospectively for 2 years in California. Twenty-six were treated with LEV and pyrazinamide (PZA) for 9 months, and 57% (15/26) completed treatment. Five children received no treatment. There was no TB incidence. While all treated children experienced adverse effects, 42% (11/26) resulted in treatment discontinuation.
Bamrah et al [19] conducted a prospective observational study of 119 persons (median age, 24 years) with MDR LTBI, with linkage to index-case molecular results, in Chuuk, Micronesia. One hundred four of the 119 persons (87%) received effective treatment with 12 months of daily moxifloxacin (MOX) or LEV alone, MOX or LEV combined with ethambutol (EMB), or LEV combined with ethionamide (ETA). Eighty-nine percent (93/104) of persons completed treatment. Persons with MDR-LTBI were followed for 3 years. There were no TB cases in treated persons and 3 (20%) TB cases in those untreated. Of the 104 persons treated, 56 (53%) experienced adverse effects (33% nausea, 25% dizziness/headache, 15% fatigue), and 4 (4%) stopped treatment due to adverse effects (2 nausea, 1 muscle/joint pain, 1 hepatitis diagnosed with acute hepatitis A).
Denholm et al [20] performed a retrospective review of medical records of contacts to infectious MDR-TB reported from 1995–2010 and molecular analysis of index-case MDR-TB isolates in Victoria, Australia. Of 570 contacts, 49 (9%; median age 27) had presumed MDR LTBI. Eleven of 49 (22%) persons with MDR-LTBI received effective treatment with 1–2 medications (mostly moxifloxacin alone or with EMB; PZA with EMB, isoniazid [INH], or rifampin [RIF]; ciprofloxacin alone or with PZA) for 6–9 months. Eighty-two percent completed treatment; 2 (18%) stopped due to adverse effects. Persons were followed for approximately 5 years. No TB cases occurred in persons treated for MDR-LTBI, and 2 (5%) TB cases occurred in untreated persons.
Schaaf et al [21] performed a prospective observational study from 1994–2000 of 105 children <5 years of age who had household contact to infectious MDR-TB in South Africa. Molecular results were unavailable for some index cases. Forty-one (39%) received effective treatment with 3–4 drug combinations of INH/PZA/EMB/ETA for 6 months. All children completed ≥6 months of treatment and were followed for 30 months. Two of 41 (5%) treated children developed TB, vs 13 of 64 (20%) untreated children. While the use of ETA caused adverse gastrointestinal effects in some, all children continued to complete at least 6 months of therapy.
Williams et al [23] retrospectively reviewed 12 children with MDR-LTBI in the United Kingdom and followed them for 2 years. Regimens varied for the 8 treated children, but included 2 effective medications for 6–12 months and all completed. Four children received no treatment. There were no incident TB cases.
After combining data from the comparison studies of Adler-Shohet et al [22], Bamrah et al [19], Denholm et al [20], Schaaf et al [21], and Williams et al [23], we found a statistically significant reduction in TB incidence among treated vs untreated persons (Figure 2). The reduction in MDR TB incidence was found using several methods (a 91%–92% risk reduction controlling for person-time using Poisson regression alone or controlling for zero inflation and random effects) to analyze count data with multiple zero outcomes. However, the best fit was a negative binomial model that controlled for person-time and overdispersion that found a 90% risk reduction, but with a very wide CI (9%–99%).
Figure 2.
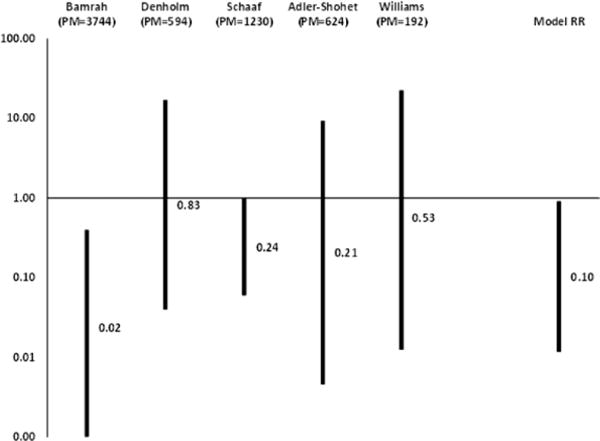
Relative risks of tuberculosis incidence after multidrug-resistant latent tuberculosis treatment compared with no treatment, from meta-analysis of 5 comparison studies. Abbreviations: PM, person-months of observation with and following treatment; RR, relative risk.
After excluding 6 of the 10 treatment-only studies that did not report TB incidence, 1 that reported incidence based solely on a registry match, and 1 that did not report methods to ascertain incidence and had 48% of patients lost to follow-up, we abstracted TB incidence results from 2 treatment-only studies [25, 31] and 5 studies on untreated persons [33–37] (Table 2). Aggregated incidence in the 3 treatment studies was 3.0% (6/210), vs 3.7% (94/2551) in the no-effective-treatment studies.
Table 2.
Tuberculosis Incidence Data from 2 Latent Tuberculosis Treatment Studies and 5 No-Effective-Treatment Studies
| Study | Tuberculosis | No | Tuberculosis Total |
|---|---|---|---|
| Treatment studies | |||
| Garcia-Prats et al [25] | 0 | 24 | 24 |
| Seddon et al [31] | 6 | 180 | 186 |
| Total | 6 | 204 | 210 |
|
| |||
| No-treatment studies | |||
| Amanullah et al [33] | 2 | 59 | 61 |
| Bayona et al [34] | 7 | 938 | 945 |
| Becerra et al [35] | 67 | 1232 | 1299 |
| Kritski et al [36] | 15 | 173 | 188 |
| Nitta et al [37] | 3 | 55 | 58 |
| Total | 94 | 2457 | 2551 |
The outcome of MDR-LTBI treatment completion was assessed for 13 studies that reported it. Mean treatment completion was 68% (428/633) (95% CI, 64%–71%) [8, 19–23, 25–31].
The mean percentage of persons experiencing adverse effects resulting in treatment discontinuation (19% [106/558]; 95% CI, 16%–22%) was aggregated from 12 studies [8, 19–22, 25–27, 29–32]. In 4 studies of children aged ≤15 years [21, 22, 25, 31], treatment discontinuation due to adverse effects averaged 5% (13/277), compared with 33% (93/281) in the remaining 8 studies. Nearly all those in the Adler-Shohet et al [22] and Schaaf et al [21] studies were treated with PZA regimens; in Garcia-Prats et al [25] and Seddon et al [31], children were treated with fluoroquinolone (FQ)/EMB regimens. The fixed relative risk (RR) of child treatment discontinuation vs adult discontinuation was 0.14 (95% CI, .08–.25).
Eleven treatment studies [19–22, 25–27, 29–32] reported data on adverse effects by treatment regimen. Regimens containing PZA had the highest percentage of adverse effects (average, 66%) and adverse effects resulting in discontinuation of MDR-LTBI treatment (average, 51%; 95% CI, 44%–59%) (Table 3). Fluoroquinolone (FQ)–containing regimens (without PZA) had a high percentage (33%) of adverse effects, but these did not often result in treatment stop (2%; 95% CI, 1%–4%). The fixed RR of treatment discontinuation using PZA-containing regimens vs FQ (excluding PZA/FQ) regimens was 27.23 (95% CI, 12.2–60.9). In children ≤15 years of age, treatment discontinuation was low with PZA/EMB (0%; 95% CI, 0%–25%) or FQ/EMB (1%; 95% CI, 0%–3%), but high with PZA/FQ (42%; 95% CI, 23%–63%).
Table 3.
Adverse Effects Reported Among Persons Receiving Treatment for Presumed Multidrug-Resistant Latent Tuberculosis by Regimen (n = 507, 11 Treatment Studies)
| Drug | No. | AEs, No. | AE Stop, No. | AEs, % | AE Stop, % |
|---|---|---|---|---|---|
| PZA/INH | 1 | 0 | 0 | 0 | 0 |
| PZA/INH/ETA | 22 | 0 | 0 | 0 | 0 |
| PZA/EMB | 14 | 9 | 7 | 64 | 50 |
| PZA/EMB/RIF | 1 | 0 | 0 | 0 | 0 |
| PZA/EMB/INH | 9 | 0 | 0 | 0 | 0 |
| PZA/EMB/INH/ETA | 2 | 0 | 0 | 0 | 0 |
| PZA/EMB/ETA | 2 | 0 | 0 | 0 | 0 |
| PZA excluding PZA/FQ regimens | 51 | 9 | 7 | 18 | 14 |
| PZA/FQ | 123 | 105 | 82 | 85 | 67 |
| PZA/FQ/INH | 6 | 4 | 4 | 67 | 67 |
| PZA/CIP | 1 | 1 | 0 | 100 | 0 |
| PZA/FQ regimens | 130 | 110 | 86 | 85 | 66 |
| All PZA regimens | 181 | 119 | 93 | 66 | 51 |
| FQ | 53 | 43 | 4 | 81 | 8 |
| FQ/EMB | 43 | 7 | 0 | 16 | 0 |
| FQ/EMB/INH | 210 | 48 | 2 | 23 | 1 |
| FQ/ETA | 12 | 7 | 0 | 58 | 0 |
| FQ regimens excluding PZA/FQ, CIP | 318 | 105 | 6 | 33 | 2 |
| CIP | 2 | 2 | 2 | 100 | 100 |
| ETA/EMB | 2 | 0 | 0 | 0 | 0 |
| ETA/EMB/INH | 4 | 0 | 0 | 0 | 0 |
| All regimens | 507 | 226 | 101 | 45 | 20 |
Abbreviations: AE, adverse effect, defined as any symptom experienced during treatment; AE stop, permanent discontinuation of treatment due to adverse effects; CIP, ciprofloxacin; EMB, ethambutol; ETA, ethionamide; FQ, fluoroquinolone; INH, isoniazid; PZA, pyrazinamide; RIF, rifampin.
We found that the most effective regimen was FQ combined with ETA; however, it was significantly (>2 times) more expensive than any other regimen and was not considered cost effective in all scenarios examined. The most cost-effective regimen was FQ/EMB, followed by FQ alone, then by PZA/ EMB (Tables 4–6). A PZA/FQ regimen was particularly toxic, as measured by treatment discontinuation, and prevented about half as many TB cases as the most cost-effective option. While we did not comprehensively estimate the costs of a no MDR-LTBI treatment option, we estimated 24 months of monitoring costs extrapolated from Holland et al [9]. There were estimated cost savings vs no treatment for all MDR-LTBI treatment regimens (because of the high societal cost [$225 000] of MDR-TB). Sensitivity analysis to assess increases in regimen adverse effects by 50% resulted in PZA/FQ becoming similar to no treatment (assumes all stop treatment), with FQ/EMB combinations still cost saving.
Table 4.
Cost-effectiveness of Multidrug-Resistant Latent Tuberculosis Treatment Regimens, 2014 US Dollars. Base Case 3% Tuberculosis Progression.
| Treatment | Estimated Regimen Efficacy, % | Estimated Stop Due to AE, % | Estimated Completion, % | Estimated US MDR-TB Cases Over 40 Remaining Years of Life, No. | TB Cases Prevented, No. | Discounted Cases Prevented, No. | Remaining Lifetime QALYs, No. | Estimated Regimen Cost, 2014 $ | Discounted Net Cost (Program Cost – Cost of TB Cases Prevented), 2014 $ | Incremental Cost (Saving) per Case Prevented, 2014 $ |
|---|---|---|---|---|---|---|---|---|---|---|
| No Tx | 480 | 0 | … | 23.6915 | $16,469,760 | |||||
| PZA/FQ | 90 | 66 | 31 | 346 | 134 | 77 | 23.6311 | $1993 | $(6,731) | saving |
| PZA/EMB | 62 | 25 | 75 | 257 | 223 | 129 | 23.6730 | $1350 | $(11,044,074) | saving |
| FQ alone | 62 | 8 | 81 | 239 | 241 | 139 | 23.6899 | $1461 | $(10,973,136) | saving |
| FQ/EMB | 76 | 1 | 79 | 192 | 288 | 167 | 23.6978 | $1893 | $(11,486,144) | saving |
| FQ/ETA | 69 | 0 | 100 | 149 | 331 | 191 | 23.6999 | $4213 | $24,264,686 | not cost effective |
For the FQ/ETA regimen, AE and treatment completion data were based on a small number of patients (n = 12).
Abbreviations: AE, adverse effect; EMB, ethambutol; ETA, ethionamide; FQ, fluoroquinolone; MDR, multidrug resistant; PZA, pyrazinamide; QALY, quality-adjusted life-year; TB, tuberculosis; Tx, treatment; US, United States.
Table 6.
Cost Effectiveness of MDR LTBI Treatment Regimens: Scenario of Low (Lower CI level) for AE Discontinuation and High (4.4%) TB Incidence
| Estimated regimen efficacy | Estimated % stop due to AE | Estimated % completion | Estimated U.S. MDR TB cases over 40 remaining years of life | TB cases prevented | Discounted cases prevented | Remaining Lifetime QALYs | Estimated regimen cost | Discounted Net Cost (Program Cost-Cost of TB Cases Prevented) | Incremental Cost (Saving) per Case Prevented | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | # | # | # | 2014$ | 2014$ | 2014$ | ||
| No Tx | 704 | 0 | – | 23.6858 | $ 16,469,760 | |||||
| PZA/FQ | 90% | 57% | 40% | 451 | 253 | 146 | 23.6380 | $ 1,993 | $ (13,716,976) | saving |
| FQ alone | 62% | 2% | 87% | 324 | 380 | 219 | 23.6935 | $ 1,461 | $ (28,104,187) | saving |
| PZA/EMB | 62% | 11% | 89% | 316 | 388 | 224 | 23.6852 | $ 1,350 | $ (30,579,993) | saving |
| FQ/EMB | 76% | 0% | 80% | 276 | 428 | 247 | 23.6966 | $ 1,893 | $ (29,498,490) | saving |
| FQ/ETA | 69% | 0% | 100% | 218 | 486 | 281 | 23.6981 | $ 4,213 | $ 4,133,156 | not cost effective |
DISCUSSION
MDR-TB is an emerging condition of public health significance. Globally, an estimated 5% of persons with TB have MDR-TB, an estimated 480 000 cases in 2014 [38]. While only 100 cases (1% of total cases) on average have been reported to the CDC each year from 2010 to 2014, MDR-TB causes greater morbidity and mortality, and patient outcomes are worse (ie, result in death, relapse, treatment failure, or disability) than for drug-susceptible TB. People exposed to MDR-TB, especially children <5 years of age or those with immunosuppression, are at great risk of progressing to this serious, often deadly airborne disease that takes years to treat. Current MDR-LTBI treatment guidelines are based on expert opinion from observational studies while clinical trial results are awaited. Several entities (CDC, American Thoracic Society, World Health Organization) are in the process of developing formal guidelines for the treatment of MDR-TB and MDR-LTBI; however, final guidance is not expected until 2018. We conducted a systematic review of published studies and found 5 studies that might be comparable. Only 1 of the 5 studies was large enough to show statistically significant results on its own. We found, by using meta-analysis of the 5 studies, empirical evidence for an approximately 90% reduction in MDR-TB incidence from MDR-LTBI treatment.
A registry match study [39] reporting results of MOX or PZA/MOX MDR-LTBI treatment regimens used in 2 outbreaks, mostly among persons with HIV, in New York City was published 3 months after completion of our systematic review, but would have been excluded from our meta-analysis because it was a registry match. Of those started on MDR-LTBI treatment, 60% (30/50) completed treatment and 6% (3/50) stopped due to adverse effects. This study found, by matching LTBI and TB registries, no related (by genotype) incident MDR-TB cases 9 years postexposure. However, many persons died or were lost to follow-up before completing evaluation for TB. The authors concluded that “contacts at greatest risk for development of disease may have done so during the outbreak investigation.”
Our review found many studies with no related MDR-TB incidence among contacts during 1–6 years of follow-up, implying little risk of developing disease, treated or not. More likely, the risk of disease is so low that small studies are not statistically powered to identify future cases. In the United States, it would take 2–5.5 years to detect 1 incident MDR-TB case (Supplementary Appendix). Attamna et al [7], Feja et al [8], and Trieu et al [39] used registry matches and found no related incident MDR-TB cases. Because MDR-TB is so rare in low-incidence settings and occurs largely in foreign-born persons, it is possible that moves/relocations resulted in no matches. Inclusion of these registry matches in our meta-analysis would have resulted in a statistically insignificant association of MDR-LTBI treatment with TB incidence.
Additional limitations include possible publication bias toward studies showing an effect, observational design of included studies, possible preference for treating highest-risk contacts (would bias against an effect), study-specific definitions of contacts and LTBI, and the lack of consistent reporting of treatment completion, adverse effects, and TB incidence by regimen within the studies. Data are and conclusions should be limited to the regimens presented. Also, with current diagnostics, the drug susceptibility of LTBI cannot be assessed.
All MDR-LTBI regimens examined were cost saving compared with no treatment with 40 remaining years of life at 3% risk of reactivation. At younger ages (more remaining years of life) or with comorbidities (or higher TB prevalence settings) that increase TB risk over 40 years to 4% or greater, FQ monotherapy becomes more cost effective. Other modeling studies also show that treatment of MDR-LTBI is cost effective: Holland et al [9] found FQ/EMB most cost effective; Fox et al [40] found FQ monotherapy cost saving. Large studies or trials are needed to fully evaluate the efficacy, effectiveness, and costs of MDR-LTBI treatment. However, while awaiting these results, the data we present are useful to guide decisions in the interim.
CONCLUSIONS
We found, using meta-analysis, empirical evidence for the effectiveness of LTBI treatment to prevent progression to MDR-TB. Very few studies met inclusion criteria, so results should be cautiously interpreted. We also found high treatment discontinuation rates due to adverse effects in persons taking PZA-containing MDR-LTBI regimens (especially PZA-FQ). This review adds to mounting evidence of the probable effectiveness and cost-effectiveness of preventing MDR-TB through treatment of MDR-LTBI and suggests that 1 or 2 drug MDR-LTBI regimens excluding PZA might be the most cost-effective.
Supplementary Material
Table 5.
Cost-effectiveness of Multidrug-Resistant Latent Tuberculosis Treatment Regimens: Scenario of High (Upper Confidence Interval Bound) for Adverse Effect Discontinuation and Low (2.4%) Tuberculosis Incidence
| Treatment | Estimated Regimen Efficacy, % | Estimated Stop Due to AE, % | Estimated Completion, % | Estimated US MDR-TB Cases Over 40 Remaining Years of Life, No. | TB Cases Prevented, No. | Discounted Cases Prevented, No. | Remaining Lifetime QALYs, No. | Estimated Regimen Cost, 2014 $ | Discounted Net Cost (Program Cost – Cost of TB Cases Prevented), 2014 $ | Incremental Cost (Saving) per Case Prevented, 2014 $ |
|---|---|---|---|---|---|---|---|---|---|---|
| No Tx | 384 | 0 | … | 23.6939 | $16,469,760 | |||||
| PZA/FQ | 90 | 74 | 23 | 305 | 79 | 46 | 23.6240 | $1993 | $5,438,976 | not cost effective |
| PZA/EMB | 62 | 45 | 55 | 253 | 131 | 76 | 23.6535 | $1350 | $(1,836,637) | saving |
| FQ alone | 62 | 18 | 71 | 215 | 169 | 98 | 23.6807 | $1461 | $(3,138,387) | saving |
| FQ/ETA | 69 | 25 | 75 | 185 | 199 | 115 | 23.6747 | $4213 | $30,317,794 | not cost effective |
| FQ/EMB | 76 | 3 | 77 | 159 | 225 | 130 | 23.6967 | $1893 | $(3,621,950) | saving |
For the FQ/ETA regimen, AE and treatment completion data were based on a small number of patients (n = 12).
Abbreviations: AE, adverse effect; EMB, ethambutol; ETA, ethionamide; FQ, fluoroquinolone; MDR, multidrug-resistant; PZA, pyrazinamide; QALY, quality-adjusted life-year; TB, tuberculosis; Tx, treatment; US, United States.
Acknowledgments
The study authors acknowledge the contributions of colleagues in the Centers for Disease Control and Prevention (CDC)/ ATS MDR Guidelines Work Group in guiding analysis; Andrew Hill and Tracy Ayers of CDC in providing recommendations for meta-analytic methods; Michael Chen for analysis of statistical power to detect incident MDR-TB; and the assistance of Michael Goodman of Emory University with systematic review methodology and analysis.
Footnotes
Financial support. This work was supported by the CDC.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Research Excellence to Stop TB Resistance. Drug-resistant tuberculosis clinical trials progress report. Available at: http://www.resisttb.org/?page_id=1602. Accessed 9 February 2017.
- 2.Centers for Disease Control and Prevention. Management of persons exposed to multidrug-resistant tuberculosis. MMWR Recomm Rep. 1992;41:61–71. [PubMed] [Google Scholar]
- 3.Curry International Tuberculosis Center and California Department of Public Health. Drug-resistant tuberculosis: a survival guide for clinicians. (3rd) :284–289. Available at: http://www.currytbcenter.ucsf.edu/products/cover-pages/drug-resistant-tuberculosis-survival-guide-clinicians-3rd-edition. Accessed June 2016.
- 4.Seddon JA, Fred D, Amanullah F, et al. Policy Brief No 1. Dubai, UAE: Harvard Medical School Center for Global Health Delivery-Dubai; 2015. Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations. [Google Scholar]
- 5.Cochrane Handbook for Systematic Reviews of Interventions. Available at: http://training.cochrane.org/handbook. Accessed October 2016.
- 6.Nuermberger E, Tyagi S, Williams KN, Rosenthal I, Bishai WR, Grosset JH. Rifapentine, moxifloxacin, or DNA vaccine improves treatment of latent tuberculosis in a mouse model. Am J Respir Crit Care Med. 2005;172:1452–6. doi: 10.1164/rccm.200507-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attamna A, Chemtob D, Attamna S, et al. Risk of tuberculosis in close contacts of patients with multidrug resistant tuberculosis: a nationwide cohort. Thorax. 2009;64:271. doi: 10.1136/thx.2008.100974. [DOI] [PubMed] [Google Scholar]
- 8.Feja K, McNelley E, Tran CS, Burzynski J, Saiman L. Management of pediatric multidrug-resistant tuberculosis and latent tuberculosis infections in New York City from 1995 to 2003. Pediatr Infect Dis J. 2008;27:907–12. doi: 10.1097/INF.0b013e3181783aca. [DOI] [PubMed] [Google Scholar]
- 9.Holland DP, Sanders GD, Hamilton CD, Stout JE. Strategies for treating latent multiple-drug resistant tuberculosis: a decision analysis. PLoS One. 2012;7:e30194. doi: 10.1371/journal.pone.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20:812–820. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro KG, Marks SM, Chen MP, et al. Estimating tuberculosis cases and their economic costs averted in the United States over the past two decades. Int J Tuberc Lung Dis. 2016;20:926–33. doi: 10.5588/ijtld.15.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bureau of Labor Statistics. (Series ID CUUR0000SAM).Consumer Price Index—all urban consumers, medical care. Available at: http://data.bls.gov/cgi-bin/srgate. Accessed 15 January 2016.
- 13.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11:1154–61. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeats J, Baker B, Basurto-Davila R. Priorities for targeted testing for latent tuberculosis infection among foreign-born adults in the United States. PhD dissertation. :2015. Available at: http://www.rand.org/content/dam/rand/pubs/rgs_dissertations/RGSD300/RGSD356/RAND_RGSD356.pdf. Accessed 14 April 2016.
- 15.Fraser A, Paul M, Attamna A, Leibovici L. Drugs for preventing tuberculosis in people at risk of multiple-drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2006(2):CD005435. doi: 10.1002/14651858.CD005435.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser A, Paul M, Attamna A, Leibovici L. Treatment of latent tuberculosis in persons at risk for multidrug-resistant tuberculosis: systematic review. Int J Tuberc Lung Dis. 2006;10:19–23. [PubMed] [Google Scholar]
- 17.Langendam MW, Tiemersma EW, van der Werf MJ, Sandgren A. Adverse events in healthy individuals and MDR-TB contacts treated with anti-tuberculosis drugs potentially effective for preventing development of MDR-TB: a systematic review. PLoS One. 2013;8:e53599. doi: 10.1371/journal.pone.0053599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Werf MJ, Langendam MW, Sandgren A, Manissero D. Lack of evidence to support policy development for management of contacts of multidrug-resistant tuberculosis patients: two systematic reviews. Int J Tuberc Lung Dis. 2012;16:288–96. doi: 10.5588/ijtld.11.0437. [DOI] [PubMed] [Google Scholar]
- 19.Bamrah S, Brostrom R, Fred D, et al. Treatment for multidrug-resistant latent tuberculosis infection—Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014;18:912–919. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denholm JT, Leslie DE, Jenkin GA, et al. Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995–2010. Int J Tuberc Lung Dis. 2012;16:1320–5. doi: 10.5588/ijtld.12.0092. [DOI] [PubMed] [Google Scholar]
- 21.Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, Donald PR. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109:765–71. doi: 10.1542/peds.109.5.765. [DOI] [PubMed] [Google Scholar]
- 22.Adler-Shohet FC, Low J, Carson M, Girma H, Singh J. Management of latent tuberculosis infection in child contacts of multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2014;33:664–6. doi: 10.1097/INF.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 23.Williams B, Ramroop S, Shah P, et al. Management of pediatric contacts of multidrug resistant tuberculosis in the United Kingdom. Pediatr Infect Dis J. 2013;32:926–7. doi: 10.1097/INF.0b013e31829157e9. [DOI] [PubMed] [Google Scholar]
- 24.Freier G, Wright A, Nelson G, et al. Multidrug-resistant tuberculosis in military recruits. Emerg Infect Dis. 2006;12:760–2. doi: 10.3201/eid1205.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Prats AJ, Zimri K, Mramba Z, Schaaf HS, Hesseling AC. Children exposed to multidrug-resistant tuberculosis at a home-based day care centre: a contact investigation. Int J Tuberc Lung Dis. 2014;18:1292–8. doi: 10.5588/ijtld.13.0872. [DOI] [PubMed] [Google Scholar]
- 26.Horn DL, Hewlett D, Jr, Alfalla C, Peterson S, Opal SM. Limited tolerance of ofloxacin and pyrazinamide prophylaxis against tuberculosis. N Engl J Med. 1994;330:1241. doi: 10.1056/nejm199404283301718. [DOI] [PubMed] [Google Scholar]
- 27.Lou HX, Shullo MA, McKaveney TP. Limited tolerability of levofloxacin and pyrazinamide for multidrug-resistant tuberculosis prophylaxis in a solid organ transplant population. Pharmacotherapy. 2002;22:701–4. doi: 10.1592/phco.22.9.701.34065. [DOI] [PubMed] [Google Scholar]
- 28.Miramontes R, Lambert L, Haddad MB, et al. Public health response to a multidrug-resistant tuberculosis outbreak among Guatemalans in Tennessee, 2007. South Med J. 2010;103:882–6. doi: 10.1097/SMJ.0b013e3181eba488. [DOI] [PubMed] [Google Scholar]
- 29.Papastavros T, Dolovich LR, Holbrook A, Whitehead L, Loeb M. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167:131–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Ridzon R, Meador J, Maxwell R, Higgins K, Weismuller P, Onorato IM. Asymptomatic hepatitis in persons who received alternative preventive therapy with pyrazinamide and ofloxacin. Clin Infect Dis. 1997;24:1264–5. doi: 10.1093/clinids/24.6.1264. [DOI] [PubMed] [Google Scholar]
- 31.Seddon JA, Hesseling AC, Finlayson H, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis. 2013;57:1676–84. doi: 10.1093/cid/cit655. [DOI] [PubMed] [Google Scholar]
- 32.Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–4. doi: 10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 33.Amanullah F, Ashfaq M, Khowaja S, et al. High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2014;18:520–7. doi: 10.5588/ijtld.13.0593. [DOI] [PubMed] [Google Scholar]
- 34.Bayona J, Chavez-Pachas AM, Palacios E, Llaro K, Sapag R, Becerra MC. Contact investigations as a means of detection and timely treatment of persons with infectious multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2003;7(12 suppl 3):S501–9. [PubMed] [Google Scholar]
- 35.Becerra MC, Franke MF, Appleton SC, et al. Tuberculosis in children exposed at home to multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2013;32:115–9. doi: 10.1097/INF.0b013e31826f6063. [DOI] [PubMed] [Google Scholar]
- 36.Kritski AL, Marques MJ, Rabahi MF, et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 1996;153:331–5. doi: 10.1164/ajrccm.153.1.8542139. [DOI] [PubMed] [Google Scholar]
- 37.Nitta AT, Knowles LS, Kim J, et al. Limited transmission of multidrug-resistant tuberculosis despite a high proportion of infectious cases in Los Angeles County, California. Am J Respir Crit Care Med. 2002;165:812–7. doi: 10.1164/ajrccm.165.6.2103109. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. MDR TB fact sheet. Available at: http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf?ua=1. Accessed 24 February 2016.
- 39.Trieu L, Proops DC, Ahuja SD. Moxifloxacin prophylaxis against MDR TB, New York, New York, USA. Emerg Infect Dis. 2015;21:500–3. doi: 10.3201/eid2103.141313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox GJ, Oxlade O, Menzies D. Fluoroquinolone therapy for the prevention of multidrug-resistant tuberculosis in contacts. A cost-effectiveness analysis. Am J Respir Crit Care Med. 2015;192:229–37. doi: 10.1164/rccm.201501-0069OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


