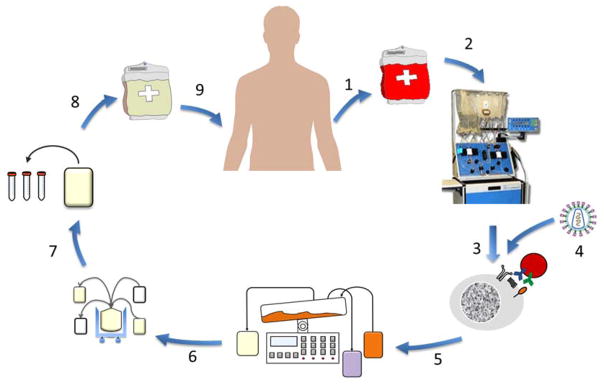
Figure 3. Engineered T Cell Manufacturing.
Leukocytes are generally collected by leukapheresis (1) and lymphocytes can be enriched (2) by counterflow centrifugal elutriation199 or subsets selected (not shown). The enriched lymphocytes are placed in to culture and (3) stimulated with bead-based artificial antigen presenting cells200,201 and viral vector (4) added202. The culture is expanded in a bioreactor for several days (5) and then the T cell bulk product (6) is washed and concentrated, samples removed for quality control release testing (7) and quality assurance review. The final formulation is cryopreserved (8), allowing facile shipment to distant infusion sites, where the final product bag (9) is thawed and infused. Manufacturing time is generally 5 to 10 days, and collection to infusion times can range from 2 to 4 weeks depending on patient clinical status and chemotherapy conditioning regimens.