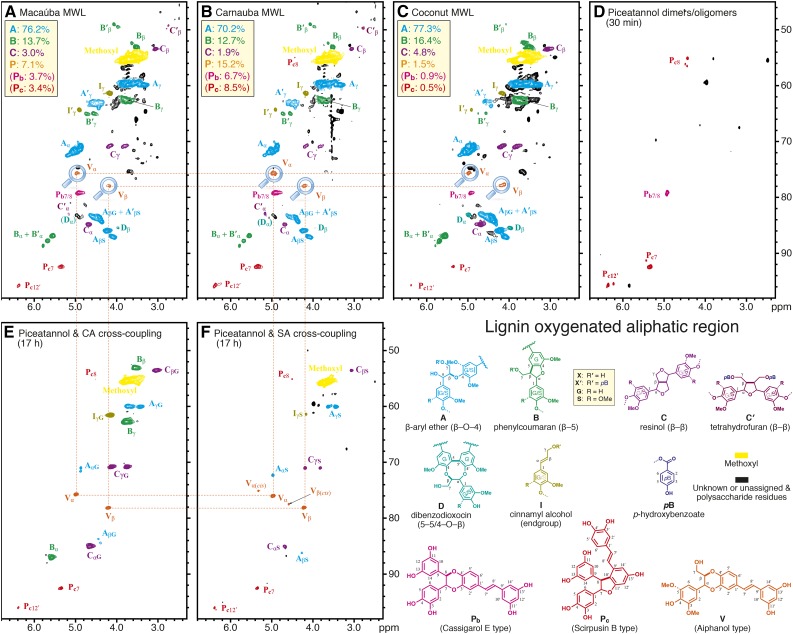
Figure 4.
Oxygenated-aliphatic regions of the 2D HSQC NMR spectra. A, Macaúba fruit endocarp MWL. B, Carnauba fruit endocarp MWL. C, Coconut endocarp MWL. D, Piceatannol dimers and oligomers showing both Pb and Pc structures with correlations that are well matched with peaks in the three lignins. E, Piceatannol and coniferyl alcohol cross-coupled polymers. F, Piceatannol and sinapyl alcohol cross-coupled polymers. The piceatannol-monolignol polymers provided evidence of the cross-coupling reactions to produce benzodioxane structures V. Both trans- and cis-configurations are evidenced in the in vitro polymerization products, but only the trans-form can be found in the lignins. Again, analogous whole cell wall spectra are shown in Supplemental Figure S2. Structures are with R = H (G unit) or R = OMe (S unit) and are labeled as X (R′ = H) or X′ (R′ = pB), where X is generic for A, B, D, and I; C′ is a special case that gets its own structure. Aromatic rings also are designated as G or S (or G/S in the case of either being allowed), with a label’s color intensity signifying the main types; for example, structures C and C′ are largely S based, with such G units being minor. Percentages for the various units are from volume integration and total 100%.