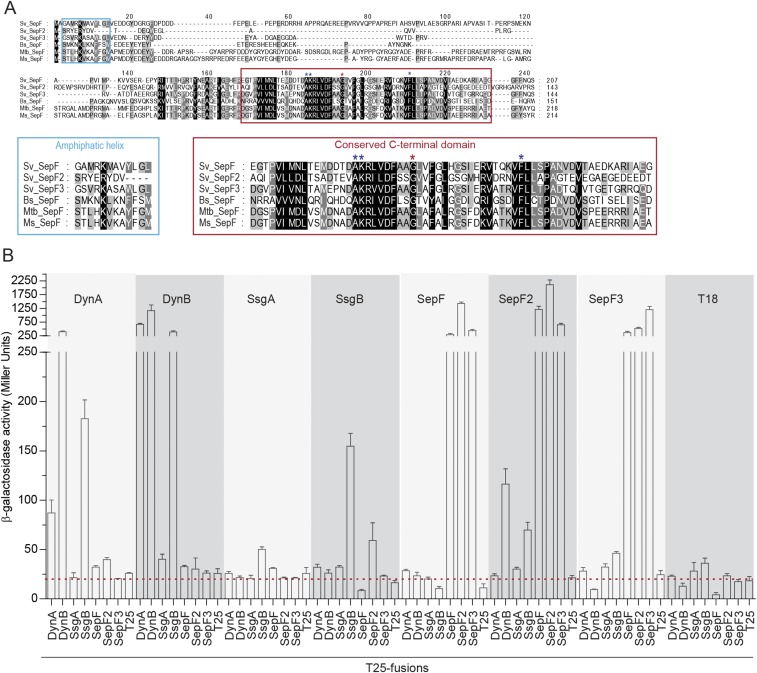
Fig. S5.
Dynamins interact with the divisome via SepF2. (A) Sequence alignment of selected SepF homologs. The conserved residues in the N terminus (blue box) and C terminus (red box) of the proteins are shown below in larger font. (Left) Sequence alignment of the residues predicted to form an amphipathic helix in the N terminus (20). Note that S. venezuelae SepF2 lacks most of the residues predicted to function in lipid binding. (Right) Sequence alignment of the C-terminal domain, containing the conserved residues responsible for SepF dimer contacts (red asterisk) and interaction with FtsZ (blue asterisks) (20, 24, 33). Bs, B. subtilis; Ms, Mycobacterium smegmatis; Mtb, M. tuberculosis; and Sv, S. venezuelae. (B) β-galactosidase activities showing the relative strengths of the interactions between the dynamins, SsgA, SsgB, and the SepF proteins. Results are the average of three independent experiments. Error bars represent the SEM. Dashed red line indicates background β-galactosidase activity as measured in the negative control (T18–T25).