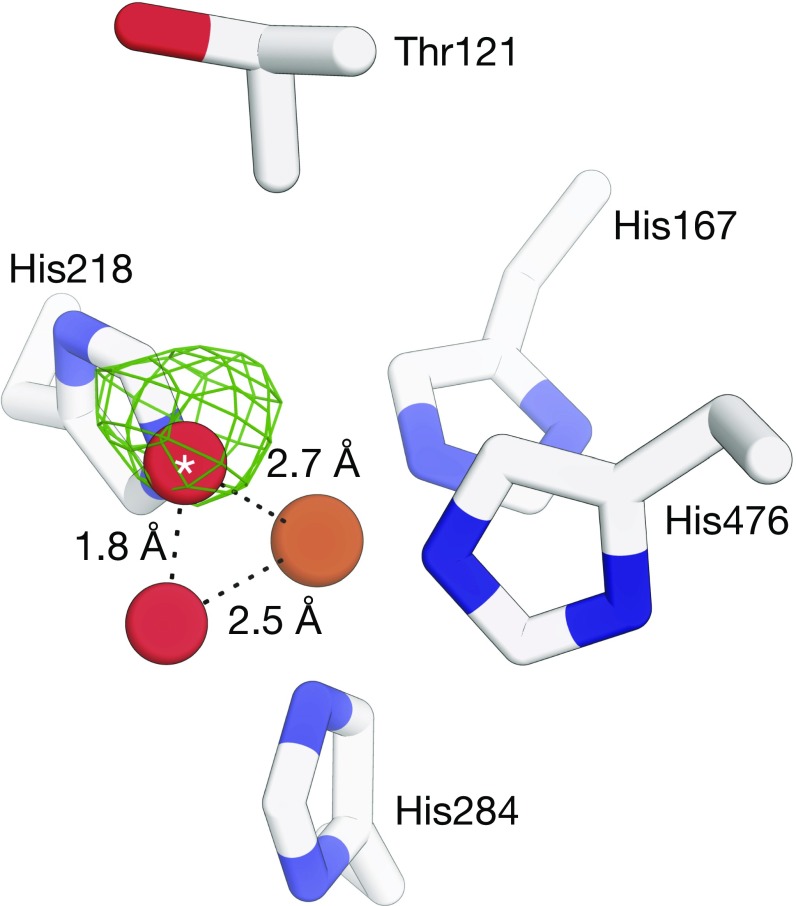
Fig. 1.
Structure of the NOV1 iron center refined without O–O and Fe–O bond-length restraints. Shown is an σA-weighted Fo − Fc electron density map (green mesh, contoured at 3 rmsd with a 3-Å carve around the dioxygen atoms) calculated with the oxygen atom of the O2 molecule (red spheres) marked with an asterisk omitted from the model. The 1.8-Å distance between the two oxygen atoms after refinement is inconsistent with a diatomic oxygen species bound to the iron center (brown sphere). Alternative refinements in which two solvent molecules were modeled and repulsive nonbonding interactions between the solvent molecules omitted from the refinement target resulted in a similar but slightly longer O–O distance. Diffraction data and starting atomic coordinates used for the refinements are deposited in the Protein Data Bank under ID code 5J53. Refinement was carried out using REFMAC version 5.8.0158.