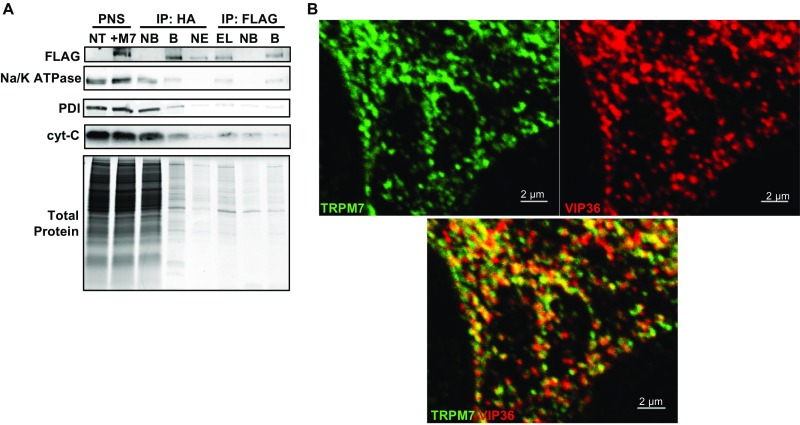
Fig. S3.
(Related to Figs. 2A and 3 and Dataset S1) Tandem affinity purification (TAP) and proteomic analysis of M7Vs. (A) Proteins of HEK293 cells expressing TRPM7 with an N-terminal FLAG-tag and cleavable C-terminal HA-tag were labeled with heavy isotopes of l-arginine and l-lysine (SILAC). After labeled cells were mixed with equal amounts of unlabeled, nontransfected wild-type (NT) cells, vesicles were isolated from the PNS using anti-HA–conjugated magnetic nanoparticles. Bound [immunoprecipitation (IP): HA, B] vesicles were eluted (IP: HA, EL) from nanoparticles using HRV-3C protease and were immunoprecipitated again with anti-FLAG–conjugated magnetic nanoparticles (IP FLAG: B). NB, not bound. TAP quality was monitored by Western blot of equal amounts of fractions for Na+/K+ ATPase, protein disulfide isomerase (PDI), cytochrome C (cyt-C), and Coomassie Blue protein staining. TAP vesicles then were analyzed by LC-MS-MS. (B) Images in Fig. 2A are presented separately to depict colocalization of VIP36-V5 and TRPM7-HA in HEK293 cells.