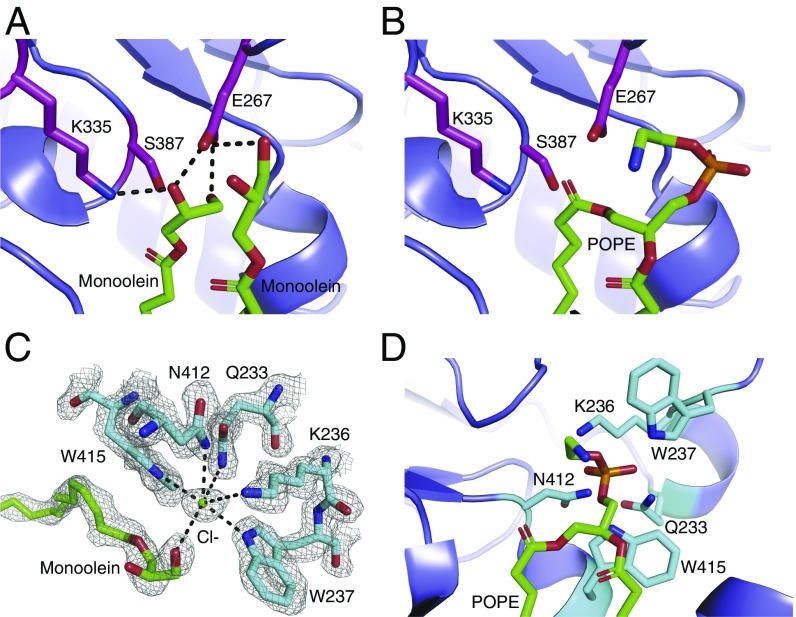
Fig. 4.
Substrate lipid recognition and catalysis by Lnt. (A) The active site of the Lnt C387S mutant. Catalytic triad residues are shown as magenta sticks. Two molecules of monoolein are shown as green sticks. Black dashed lines denote hydrogen bonds. (B) Model of POPE bound in the Lnt C387S mutant active site. Catalytic residues are shown as magenta sticks, and POPE is shown as green sticks. (C) Putative Lnt phosphate-binding site with a chloride ion bound. A monoolein molecule is shown as green sticks. Lnt residues that are coordinating the chloride ion are shown as cyan sticks. The gray mesh represents a feature-enhance electron density map contoured at 1 σ. (D) Model of POPE bound to the Lnt C387S mutant active site with the phosphoryl group positioned in the putative phosphate-binding site. POPE is shown as green sticks, and binding-site residues are shown in cyan stick representation.