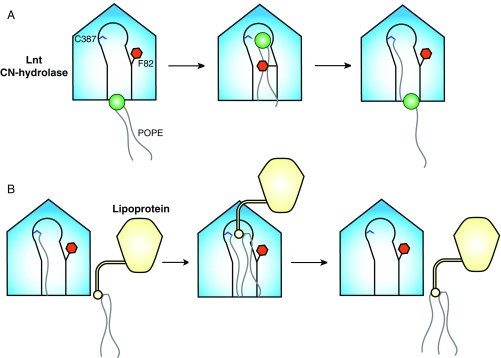
Fig. 6.
Model of lipoprotein N-acylation by E. coli Lnt. (A) Formation of an acyl-enzyme intermediate. Loop 2 of apo Lnt is highly flexible, with the gatekeeper F82 in the open position some portion of the time. A molecule of POPE first binds to the lipid-binding groove, which causes F82 to close, placing the sn-1-acyl chain carbonyl in position for nucleophilic attack by C387. This reaction forms a S-palmitoyl cysteine-modified form of Lnt. The F82 gate then opens, allowing the lyso-PE byproduct to exit. (B) Lipoprotein N-acylation. The F82 gate is mobile in the acyl-enzyme intermediate form of Lnt. This allows entrance of one acyl chain of an S-diacylated lipoprotein substrate. Entry of the S-diacylglyceryl cysteine into the vacant lipid channel positions the α-amino group of the lipoprotein N-terminal cysteine for nucleophilic attack on the carbonyl of the S-palmitoyl cysteine of Lnt. This mature, triacylated lipoprotein then exits for translocation to the OM by the Lol machinery.