Abstract
Purpose
This systematic review summarizes the effects of isometric lingual strength training on lingual strength and swallow function in adult populations. Furthermore, it evaluates the designs of the reviewed studies and identifies areas of future research in isometric lingual strength training for dysphagia remediation.
Method
A comprehensive literature search of 3 databases and additional backward citation search identified 10 studies for inclusion in the review. The review reports and discusses the isometric-exercise intervention protocols, pre- and postintervention lingual-pressure data (maximum peak pressures and lingual-palatal pressures during swallowing), and oropharyngeal swallowing measures such as penetration-aspiration scales, oropharyngeal residue and duration, lingual volumes, and quality-of-life assessments.
Results
Studies reported gains in maximum peak lingual pressures following isometric lingual strength training for both healthy adults and select groups of individuals with dysphagia. However, due to the variability in study designs, it remains unclear whether strength gains generalize to swallow function.
Conclusion
Although isometric lingual strength training is a promising intervention for oropharyngeal dysphagia, the current literature is too variable to confidently report specific therapeutic benefits. Future investigations should target homogenous patient populations and use randomized controlled trials to determine the efficacy of this treatment for individuals with dysphagia.
It is increasingly the case that therapeutic exercise programs used to treat dysphagia include isometric lingual strength training (Husaini et al., 2014). In the healthy adult swallow, the tongue exerts a force on the bolus, setting into motion a series of physiologic events that are all needed for the swallow to unfold in a coordinated, highly timed fashion (Dodds, 1989). Recent research suggests that in select etiologic groups, improvements in lingual strength can result in favorable therapeutic outcomes for individuals diagnosed with dysphagia (Juan et al., 2013; Malandraki et al., 2012; Robbins et al., 2007; Steele et al., 2013; Yeates, Molfenter, & Steele, 2008). Principles that underlie lingual strength training have their basis in exercise physiology (Kays & Robbins, 2006), although it is undetermined whether the tongue musculature responds to exercise in the same way as larger skeletal muscles. Moreover, training protocols can vary, yielding results that are not always directly comparable. Thus, clarity is needed to best understand how isometric lingual strength-training exercises can be used to treat individuals with dysphagia. The purpose of this systematic review is to summarize the existing literature on isometric lingual strength-training programs in adult populations and identify future directions for research.
Isometric Lingual Strength Training
Isometric exercise consists of “sustained contraction against an immovable load or resistance with no or minimal change in length of the involved muscle group” (Carlson, Dieberg, Hess, Millar, & Smart, 2014, p. 2). In the tongue, placement of the tongue blade directly behind the alveolar ridge is the target for anterior elevation exercise, whereas posterior tongue targets refer to placing the dorsum of the tongue against the intersection of the hard and soft palate (Clark & Solomon, 2012). Moving the tongue laterally as well as protruding forward against resistance can also be included in isometric lingual-strengthening exercise programs (Clark, O'Brien, Calleja, & Corrie, 2009). The purpose of each of these isometric exercises is to improve overall lingual strength, which is hypothesized to relate directly to lingual-palatal pressure generation and bolus propulsion during the swallow (Kays & Robbins, 2006; Robbins et al., 2005; Yeates et al., 2008).
Isometric exercise was first developed in the exercise-physiology domain to improve skeletal-muscle strength, and recently the same exercise principles have been applied to strengthening the lingual musculature (Kays & Robbins, 2006). However, tongue histology, morphology, and biomechanical properties differ considerably from those of skeletal muscle. For example, skeletal muscle is organized in long parallel fibers, whereas lingual fibers are parallel, perpendicular, and oblique in arrangement (Kier & Smith, 1985; Miller, Watkin, & Chen, 2002). Furthermore, the type and density of muscle fibers are not necessarily comparable between lingual and skeletal muscle (Miller et al., 2002; Stål, Marklund, Thornell, De Paul, & Eriksson, 2003). Whether the tongue can be strengthened in the same manner as skeletal muscles and whether strengthening exercises change the underlying arrangement, distribution, and composition of lingual muscle fibers, and thus functional muscle strength, are questions raised by clinicians and researchers.
Lingual Pressures in Healthy Adults
Previous studies have documented maximum peak lingual pressures in healthy adults, producing results that indicate lingual pressures may vary by age, sex, and tongue-palatal targets (Crow & Ship, 1996; Nicosia et al., 2000; Todd, Lintzenich, & Butler, 2013; Vanderwegen, Guns, Van Nuffelen, Elen, & De Bodt, 2013; Youmans & Stierwalt, 2006; Youmans, Youmans, & Stierwalt, 2009). A recent systematic review and meta-analysis summarized the impacts of gender, age, and tongue-palatal targets during maximum peak pressure generation in the tongue (Adams, Mathisen, Baines, Lazarus, & Callister, 2013). Results indicated that mean peak pressure values range from 43 to 78 kilopascals (kPa) for both anterior and posterior target placements in healthy adults, though maximum anterior pressures seem to be, on average, greater than posterior pressures. The meta-analyses further revealed significantly higher maximum peak pressures in male compared with female subjects, and greater lingual pressures in adults under the age of 60 years compared with older adult cohorts.
Bolus viscosity has been identified as a factor affecting lingual-palatal pressure generation during the swallow. Overall, foods of higher viscosity require greater lingual pressure during the swallow than foods of lower viscosity (Miller & Watkin, 1996; Youmans & Stierwalt, 2006). Anterior lingual pressures are greater than posterior pressures during dry swallows and across varying liquid consistencies (Gingrich, Stierwalt, Hageman, & LaPointe, 2012; Todd et al., 2013), but solid boluses require greater posterior pressure generation when compared with anterior, presumably for bolus propulsion into the pharynx (Nicosia et al., 2000).
Lingual-palatal pressures generated during swallows are considerably lower than the isometric maximum peak pressures that healthy individuals are capable of producing (Gingrich et al., 2012; Nicosia et al., 2000; Todd et al., 2013; Youmans et al., 2009; Youmans & Stierwalt, 2006). Swallowing is therefore considered a submaximal-force lingual task; that is, forces needed during swallowing are only a percentage of maximum peak pressure. Youmans and Stierwalt (2006) calculated the percentage of maximum peak pressure for the anterior tongue during thin and honey-thick liquid swallows, with a result of 51.33%. Therefore, healthy participants used approximately half of their maximum lingual strength ability during liquid swallows. In this regard, clarity is needed to determine whether there is any evidence that an isometric exercise would generalize to the submaximal, dynamic task of swallowing.
Intervention Protocols and Target Populations
The dose-related events of timing (when to begin intervention), frequency (the number of days per week), repetition (the number per day), intensity (the amount of force resistance during exercise), and duration (the length of training program) are factors in the success of any exercise program. The American College of Sports Medicine (2009) recommends that healthy adult beginners complete one to three sets of an exercise, 2–3 days a week, for isometric skeletal-muscle strengthening programs. Then, the frequency, number of repetitions, and intensity of exercise should vary on an individual basis. Lingual-strengthening protocols have borrowed from these recommendations and adapted them for lingual-strengthening programs (Kays & Robbins, 2006). For example, a progressive overload technique has been used to improve lingual strength, where there is a “gradual increase of stress placed on the body during exercise training” (American College of Sports Medicine, 2009, p. 688). A range of lingual-pressure values that are a percentage of individual maximum ability are targeted during exercises (i.e., 80% of personal maximum ability). But to date, isometric lingual strength-training programs have varied with regard to lingual placement (anterior vs. posterior), frequency, number of repetitions, and intensity of the exercise. No established standard protocol currently exists for isometric lingual strength-training programs.
In addition, concerns exist in comparing treatment outcomes across heterogeneous patient populations (Kays & Robbins, 2006). Etiologic correlates associated with decreased lingual strength include cerebrovascular accident (CVA; Hori, Ono, Iwata, Nokubi, & Kumakura, 2005; Konaka et al., 2010), muscular dystrophy (Hamanaka-Kondoh et al., 2014; Palmer, Neel, Sprouls, & Morrison, 2010), Parkinson's disease (Unemoto, Tsuboi, Kitashima, Furuya, & Kikuta, 2011), and oropharyngeal cancer (Lazarus, 2006; Lazarus et al., 2000). Although individuals in these diagnostic categories are potential candidates for lingual strength training, their outcomes are likely variable given factors related to the underlying nature of their diagnoses (e.g., remitting, progressive) and any concomitant interventions needed for disease treatment (e.g., radiation therapy). Moreover, studies examining the typical range of lingual pressures recorded for healthy adults have documented a decline in maximum peak pressure in adults over the age of 60 years (Adams et al., 2013; Todd et al., 2013; Vanderwegen et al., 2013). Although these findings suggest that healthy older adults may benefit from lingual-strengthening exercises, the need for improvement is not known, nor is the potential for these exercises to improve lingual strength.
Objectives of the Systematic Review
There is currently no available evidence-based statement on whether isometric lingual strength training is an effective intervention for dysphagia remediation, nor is there any such statement supporting a standard protocol for the population of individuals with dysphagia as a whole or by etiologic category. Thus, this systematic review sought to examine the existing evidence to (a) identify isometric lingual-strengthening programs and discuss their intended benefits for maximum peak lingual-pressure measures, (b) determine if isometric lingual-strengthening exercises (in isolation) also increase functional lingual strength during swallowing, (c) discuss oropharyngeal swallow measurements (e.g., penetration-aspiration scales, oropharyngeal residue and duration measures, lingual volumes, and quality-of-life assessments) that may have changed following isometric lingual-strengthening programs, (d) compare the range of research designs, and (e) identify directions for future lingual-strengthening research for dysphagia remediation.
Method
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Group provides crucial guidelines for the development, reporting, and replicability of systematic review methodology. We followed the guidelines established in the PRISMA statement, including adherence to the 27-item checklist and recommendations for transparent reporting (Liberati et al., 2009; Moher, Liberati, Tetzlaff, Altman, & the PRISMA Group, 2009).
Search Strategy and Eligibility Criteria
A systematic literature search was conducted across three electronic databases: Science Citation Index Expanded, Science Direct, and PubMed. The database search ranged from the year 1965 to September 2015 and was not restricted by language. The Science Citation Index Expanded includes only publications from 1965 onward, so using this time period assured continuity across searches. Search terms included tongue or lingual, AND, strength* and exercise (see Appendix A for complete list of search strings). Next, a backward citation search of a recent review and meta-analysis on lingual strength assisted in identification of additional articles (Adams et al., 2013). In order to be considered for the review, articles needed to meet the following inclusion criteria:
Human participants, age 18 years and above
Completion of a systematic, isometric lingual-strengthening program
Use of a commercially available tool to obtain lingual-pressure measurements
Assessment and reporting of pre- and posttreatment lingual pressures (raw values or comparison statistics accepted)
Length of intervention program, medical status of the adult, and gender distribution were not considered as factors for inclusion in the review.
The first and third authors independently completed database and backward citation searches. Records were first screened by title and abstract in order to determine potential eligibility on the basis of the inclusion criteria. Records satisfying these criteria were read in full to determine final eligibility. Agreement between independent searches was high, with only one article warranting further discussion on whether it met the inclusion criteria for the review.
Bias Assessment
Bias assessments evaluate the internal and external validity of study designs and reporting methods (see Table 1; Liberati et al., 2009). Due to the variation in study designs identified in this review (e.g., randomized controlled trials, case-study designs), the bias-assessment criteria were a subset of quality-assessment measures described by Hoy et al. (2012). In addition to the first six criteria, we added an assessment point to describe whether or not the study was adequately powered (an estimate of whether the sample size was large enough to determine significance). The first and fourth authors collaboratively determined the answers to the seven criterion questions using a binary (yes/no) scale:
Table 1.
Bias assessment.
| Criterion | Clark (2012) | Clark et al. (2009) | Juan et al. (2013) | Lazarus et al. (2003) | Lazarus et al. (2014) | Oh (2015) | Robbins et al. (2005) | Robbins et al. (2007) | Steele et al. (2013) | Yeates et al. (2008) | Quality summary (%) a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| External validity | |||||||||||
| Representative sample | − | + | − | + | + | − | + | + | − | − | 50 |
| Minimal nonresponse bias | + | + | + | + | − | + | − | + | + | + | 80 |
| Internal validity | |||||||||||
| Acceptable case definition | + | + | + | + | + | + | + | + | + | + | 100 |
| Valid/reliable instrument | + | + | + | + | + | + | + | + | + | + | 100 |
| Uniform data collection | + | + | + | + | + | + | + | + | + | − | 90 |
| Appropriate program length b | + | + | + | + | + | + | + | + | + | + | 100 |
| Adequate power | − | + | − | + | + | + | + | + | − | − | 60 |
Note. + = The study met the criterion. − = The study did not meet the criterion or did not provide enough information.
Percentage of studies that met the quality criterion.
Appropriate program length was determined on the basis of the typical length of dysphagia intervention protocols (Kays & Robbins, 2006) and recommendations for isometric skeletal-muscle strengthening programs (American College of Sports Medicine, 2009).
Was the sample representative of the target population?
Was there minimal chance of nonresponse bias?
Was there an acceptable case definition for participants?
Did the study use valid/reliable instrumentation for data collection?
Was data collection uniform across participants?
When compared with recommendations in peer reviewed publications, was the length of the strengthening program appropriate?
Was the study adequately powered?
A study received a positive rating for each criterion met. Any ambiguity in reporting or design structure led to a negative rating. Table 1 lists the results of the bias assessment.
Data Extraction
The first author extracted data for the review, and the third author checked all data points. Articles were re-referenced and discussed to resolve any discrepancies. When necessary, authors of the articles were contacted to confirm sample sizes, clarify demographic information, and/or request raw data and more precise statistical values. If authors could not be reached, the pressure conditions in question were not included in the review (e.g., swallow-pressure data; Juan et al., 2013).
Data extracted from the articles included raw lingual-pressure values and standard deviations (both before and after intervention), p values from pre/post comparisons, and effect size values (Cohen's d). Although all of the studies included in the systematic review assessed lingual strength, not all of those data were extracted for use. The research question was solely concerned with isometric strengthening of the tongue, because it is hypothesized that this exercise directly relates to lingual function during the oropharyngeal swallow. Therefore, maximum peak pressure values for lingual elevation (anterior and posterior), protrusion, and lateralization were only included when the article indicated that their participants specifically trained on exercises targeting those movements. Any other measures related to endurance, power, or speed of the tongue were not included in this review, nor were measures reflecting the ability to generalize specific lingual strength-training tasks to other untrained directional exercises.
Data were collected for swallows of varying textures (including saliva swallows), as well as measures reflecting oropharyngeal swallow function. These additional measurements were captured via videofluoroscopic swallow studies, magnetic resonance imaging (MRI), and standardized questionnaires. Outcomes included penetration-aspiration rating scales, oropharyngeal-residue rating scales, oropharyngeal durational measures, lingual volumes, and standardized quality-of-life assessments.
Results
Study Selection
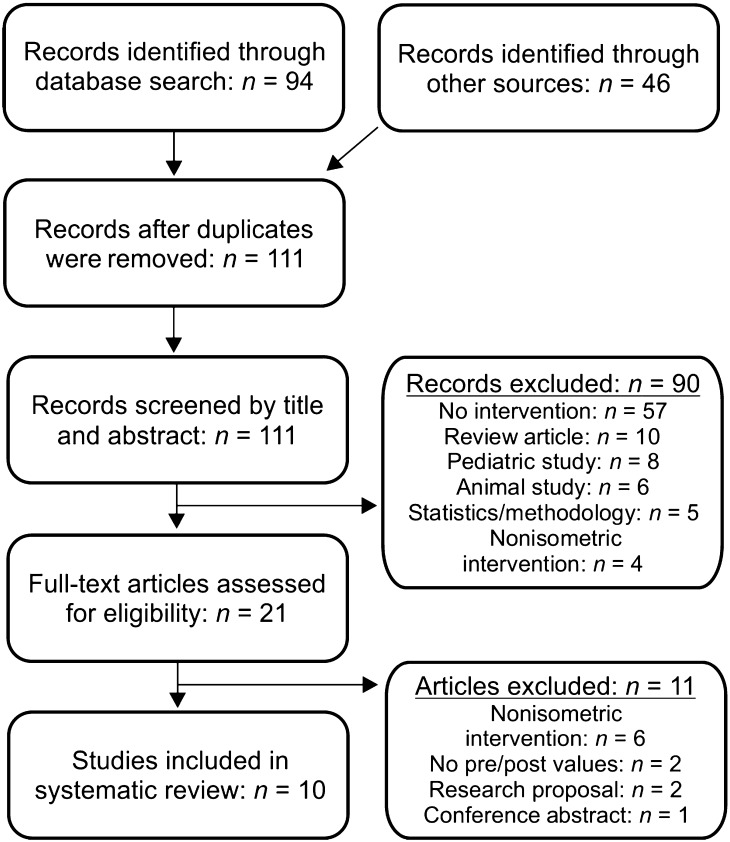
A search of three databases (Science Citation Index Expanded, Science Direct, and PubMed) and backward citation search (Adams et al., 2013) ultimately identified 10 studies for inclusion in the systematic review (see Figure 1). In order to gather additional study information, it was necessary to contact study authors. Their unpublished work provided data from additional participants for three studies (Steele, Bressmann, & Carnahan, 2007–2010; Steele, Molfenter, Bailey, Oshalla, & Yeates, 2011; Yeates et al., 2008). Appendixes B and C include the data from these unpublished works, and a separate discussion on these findings is contained in the section Publication Bias: Unpublished Data.
Figure 1.
Study selection flowchart.
Study Characteristics
Of the 10 studies selected for review, four were randomized controlled trials, three were prospective cohort interventions, and the remaining three used case-study descriptions of individuals with varying types of dysphagia (see Table 2). Five articles reported on healthy adult participants with no history of dysphagia, whereas the other five had a diverse group of individuals with dysphagia, including diagnoses of closed head injury, CVA, brain tumor, and oropharyngeal cancer. Overall, treatment groups included one to 37 participants and control groups included five to 10 total participants (see Table 3). Participant ages ranged from 18 to 90 years.
Table 2.
Pre/post measures for maximum peak lingual pressures: Treatment groups.
| Study | Participant status | Age (years) | Instrument | n | Training days | Bulb placement | Pre (SD; kPa) | Post (SD; kPa) | p value | Effect size (d) | Study design and funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clark (2012) a | Healthy | 19–57 | IOPI | 5 | 12 | Anterior | 65.8 (14.97) | 82.6 (13.39) | 1.06 | Randomized controlled trial; internal funding | |
| Clark et al. (2009) a | Healthy | 18–67 | IOPI | 37 | 63 | Anterior | < .001 | Randomized controlled trial; internal funding | |||
| Protrusion | < .001 | ||||||||||
| Lateralization | < .001 | ||||||||||
| Juan et al. (2013) | CVA | 59 | MOST | 1 | 24 | Anterior | 33 | 49 | Case study; Veterans Affairs funding | ||
| Posterior | 23 | 39 | |||||||||
| Left | 18 | 22 | |||||||||
| Right | 12 | 23 | |||||||||
| Lazarus et al. (2003) a | Healthy | 20–29 | IOPI | 21 | 20 | Anterior | 64.4 | 73.1 | < .001 | Randomized controlled trial; nonprofit-organization funding | |
| Lazarus et al. (2014) a | Oropharyngeal cancer | 50–79 | IOPI | 8 | 30 | Anterior | 44.63 (13.39) | 46.5 (16.5) | .571 | Randomized controlled trial; government funding | |
| Oh (2015) | Healthy | 21–35 | IOPI | 10 | 24 | Anterior | 64.5 (13.05) | 80.5 (12.23) | .000 | Prospective cohort intervention; internal funding | |
| Posterior | 60.8 (11.85) | 76.4 (11.11) | .000 | ||||||||
| Robbins et al. (2005) a | Healthy | 70–89 | IOPI | 10 | 18 | Anterior | 41 | 49 | .001 | Prospective cohort intervention; no funding | |
| Robbins et al. (2007) a | CVA | 51–90 | IOPI | 10 | 24 | Anterior | 35.6 | 51.8 | < .001 | Prospective cohort intervention; no funding | |
| 9 | Posterior | 30.2 | 54.6 | < .001 | |||||||
| Steele et al. (2013) a | CHI | 45 | IOPI | 6 | 24 | Anterior | 28.4 (2.7) | >0.6 | Case study; public and government funding | ||
| Posterior | 26.6 (3.6) | <0.6 | |||||||||
| 32 | Anterior | 43.5 (7.5) | >0.6 | ||||||||
| Posterior | 26.8 (5.2) | >0.6 | |||||||||
| 47 | Anterior | 23.7 (5.7) | >0.6 | ||||||||
| Posterior | 17.2 (5.1) | >0.6 | |||||||||
| 54 | Anterior | 41.9 (3.8) | >0.6 | ||||||||
| Posterior | 21.4 (2.6) | >0.6 | |||||||||
| 32 | Anterior | 28.4 (9.2) | >0.6 | ||||||||
| Posterior | 23.3 (7.6) | >0.6 | |||||||||
| 44 | Anterior | 32.4 (8.6) | <0.6 | ||||||||
| Posterior | 20.3 (9.1) | >0.6 | |||||||||
| Yeates et al. (2008) a | CVA | 72 | IOPI | 3 | 24 | Anterior | 48 (6.03) | 69.75 (2.6) | Case study; public and government funding | ||
| Posterior | 52.92 (4.29) | 69.92 (2.73) | |||||||||
| Brain tumor | 63 | 24 | Anterior | 20.33 (0.48) | 55.89 (3.28) | ||||||
| Posterior | 26 (4.09) | 50 (4.04) | |||||||||
| MVA & CVA | 50 | 90 | Anterior | 29.20 (3.14) | 37.06 (1.47) | ||||||
| Posterior | 22.67 (2.13) | 34.56 (0.92) | |||||||||
| 138 | Anterior | 61.83 (4.22) | |||||||||
| Posterior | 56.67 (4.73) |
Note. IOPI = Iowa Oral Performance Instrument; CVA = cerebrovascular accident; MOST = Madison Oral Strengthening Therapeutic device; CHI = closed head injury; MVA = motor-vehicle accident.
Authors provided further study information.
Table 3.
Pre/post measures for maximum peak lingual pressures: Control groups.
| Study | Participant status | Exercise regimen | Age (years) | n | Instrument | Training days | Bulb placement | Pre (SD; kPa) | Post (SD; kPa) | p value | Effect size (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clark (2012) | Healthy | None | 19–57 | 5 | IOPI | 0 | Anterior | 66.8 (13.18) | 73.6 (10.06) | 0.52 | |
| Lazarus et al. (2003) | Healthy | None | 20–29 | 10 | IOPI | 0 | Anterior | 69.8 | 71.2 | .62 | |
| Lazarus et al. (2014) | Oropharyngeal cancer | Traditional exercise | 50–79 | 10 | IOPI | 30 | Anterior | 49.3 (10.53) | 52.4 (10.78) | .335 |
Note. IOPI = Iowa Oral Performance Instrument.
All 10 studies reported isometric lingual-strength outcomes (as stated in the inclusion criteria), but less than half reported outcome data from lingual-palatal pressure generation during swallowing (see Table 4; Oh, 2015; Robbins et al., 2005, 2007; Steele et al., 2013). The Iowa Oral Performance Instrument (IOPI Medical, Redmond, WA) was the most commonly used instrument for collecting maximum peak lingual-pressure data; however, the Pentax Digital Swallow Workstation (Pentax Medical, Montvale, NJ) and Iowa Oral Performance Instrument were both used to gather data during swallowing. The study by Juan et al. (2013) used the Madison Oral Strengthening Therapeutic device (Swallow Solutions, Madison, WI). This device is the only instrument to measure lateral tongue pressures in addition to anterior and posterior pressures; thus, there are few data points reported for the lateral edges of the tongue in this review.
Table 4.
Pre/post measures for lingual-palatal pressure during swallows: Treatment groups.
| Study | Participant status | Age (years) | Instrument (unit) | n | Training days | Bulb placement | Assessment | Pre | Post | p value | Effect size (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oh (2015) | Healthy | 21–35 | IOPI (kPa) | 10 | 24 | Posterior | Saliva, effortful | 53.2 | 71.6 | .000 | |
| Robbins et al. (2005) | Healthy | 70–89 | DSW (mm Hg) | 10 | 24 | Peak pressure a | 3 mL thin | .18 | |||
| 10 mL thin | .04 | ||||||||||
| 3 mL semisolid | .01 | ||||||||||
| 3 mL thin, effortful | .001 | ||||||||||
| Robbins et al. (2007) | CVA | 51–90 | DSW (mm Hg) | 7 | 24 | Peak pressure a | 3 mL thin, effortful | 155.72 | 163.99 | .62 | |
| 146.41 | 154.73 | .53 | |||||||||
| 10 mL thin | 53.43 | 105.21 | .03 | ||||||||
| 57.48 | 105.23 | .07 | |||||||||
| 56.42 | 97.82 | .20 | |||||||||
| 3 mL semisolid | 91.03 | 126.11 | .02 | ||||||||
| 100.14 | 135.42 | .06 | |||||||||
| 123.14 | 152.33 | .14 | |||||||||
| 3 mL thin | 84.29 | 127.56 | .04 | ||||||||
| 68.58 | 141.60 | .004 | |||||||||
| 118.17 | 118.85 | .97 | |||||||||
| Steele et al. (2013) | CHI | 45 | IOPI (kPa) | 6 | 24 | Anterior | Saliva swallow | >0.6 | |||
| 32 | <0.6 | ||||||||||
| 47 | <0.6 | ||||||||||
| 54 | <0.6 | ||||||||||
| 32 | <0.6 | ||||||||||
| 44 | <0.6 |
Note. IOPI = Iowa Oral Performance Instrument; DSW = Digital Swallow Workstation; CVA = cerebrovascular accident; CHI = closed head injury.
Three-bulb array measuring anterior/middle/posterior where the greatest value at any position is reported.
Training and Intervention Protocols
Exercise programs varied considerably in the frequency, intensity, and duration of treatment. Intervention doses ranged from two to seven times per week, and the duration of treatment typically lasted 4–9 weeks, with the design by Steele et al. (2013) ranging longer, from 11 to 12 weeks. The number of individual training days varied from 12 to 138, with a mode of 24 training days per study. Only one participant continued intervention for 138 training days as part of a personal dysphagia-intervention program (Yeates et al., 2008). Otherwise, the study by Clark et al. (2009) had the next largest total, with 63 days of training per participant.
The number of exercise repetitions completed each session ranged from 10 to 60 per target, with practice sessions occurring one to five times per day. Throughout repetition tasks, studies typically included multiple targets for exercise (e.g., anterior and posterior tongue positions), with the exception of two studies that practiced using the anterior tongue position only (Clark, 2012; Robbins et al., 2005).
Target pressures ranged from as low as 20% of personal maximum peak pressure (Steele et al., 2013) to as high as 100% of personal maximum peak pressure during practice (Clark et al., 2009; Lazarus et al., 2014; Lazarus, Logemann, Huang, & Rademaker, 2003). Other studies started out at lower target levels (e.g., 60% of maximum peak ability) and increased to 80% of personal maximum peak pressure as the strength of the individual improved (Juan et al., 2013; Oh, 2015; Robbins et al., 2005, 2007).
Maximum Peak Lingual Pressures
Initial strength values ranged from 41 to 65 kPa in healthy cohorts and 12 to 52 kPa in participants with dysphagia. Posterior lingual pressures at baseline were consistently lower than anterior lingual pressures for all participants, congruent with results from prior reports (Adams et al., 2013). Except for a limited few, participants with dysphagia had baseline lingual-pressure measures that fell below the established normative range for position and age (Adams et al., 2013; Nicosia et al., 2000).
Five of six studies reported statistically significant gains via t-test comparisons of lingual strength at the site of exercise (Clark et al., 2009; Lazarus et al., 2003; Oh, 2015; Robbins et al., 2005, 2007). Only the study by Lazarus et al. (2014) reported nonsignificant findings. In that study, investigators evaluated two groups of participants diagnosed with oropharyngeal cancer. The control group completed traditional pharyngeal-strengthening exercises (e.g., Mendelsohn maneuver), and the treatment group completed both traditional and isometric lingual-strengthening exercises. The authors found no significant differences for maximum peak lingual pressures within or between the treatment and control groups following intervention.
Two studies described treatment outcomes for maximum peak lingual measures in terms of effect size (Cohen's d). Clark (2012) found a large effect size for pre/post maximum peak lingual-strength comparisons for five healthy participants (d = 1.06). Using a case-study design, Steele et al. (2013) calculated individual effect size bands (d = 0.6), reporting changes > 0.6 in 10 out of 12 maximum lingual-pressure comparisons.
Lingual-Palatal Pressures During Swallowing
Four studies assessed lingual-palatal pressure changes during swallowing tasks (see Table 4) by capturing lingual pressures during saliva swallows, thin liquids, effortful swallows, and semisolid viscosities of varying amounts. Results across studies were inconsistent. For example, in one study, evaluation of 11 different swallows found only four to have significantly increased lingual-palatal pressures following intervention, but these increases were not specific to any single consistency or effort/noneffort condition (Robbins et al., 2007). In the study by Steele et al. (2013), similarly, only one participant showed a medium effect size difference in a pre/post lingual-pressure comparison of saliva swallows. In older healthy participants, t-test comparisons revealed more consistent changes showing significant differences for three out of four swallow conditions (Robbins et al., 2005), and the most promising statistical changes were reported in young, healthy adults (Oh, 2015).
Oropharyngeal Swallow Measures
Oropharyngeal swallow measures were reported in studies with both healthy adults and those diagnosed with dysphagia (see Table 5). As expected, healthy adults did not exhibit any change in oropharyngeal measures, aside from lingual volume, because they had no history of dysphagia and most likely had functional swallows at baseline (Robbins et al., 2005). The following subsections are dedicated to describing the swallowing outcomes as they pertain to those participants diagnosed with dysphagia, though healthy older adult participants are included in the lingual-volume summary as well.
Table 5.
Oropharyngeal swallow measures.
| Study | Penetration-Aspiration scale | Oropharyngeal residue | Oropharyngeal duration | Lingual volume | Quality-of-life questionnaires |
|---|---|---|---|---|---|
| Clark (2012) | |||||
| Clark et al. (2009) | |||||
| Juan et al. (2013) | X | X | X | X | X |
| Lazarus et al. (2003) | |||||
| Lazarus et al. (2014) | X | X | X | ||
| Oh (2015) | |||||
| Robbins et al. (2005) | X | X | X | X | |
| Robbins et al. (2007) | X | X | X | X | X |
| Steele et al. (2013) | X | X | |||
| Yeates et al. (2008) |
Penetration-Aspiration Scale
Swallows captured via videofluoroscopy were evaluated using the 8-point Penetration-Aspiration Scale (PAS) to identify the presence and degree of any prandial material within the airway (Rosenbek, Robbins, Roecker, Coyle, & Woods, 1996). Robbins et al. (2007) reported positive results, noting a reduced frequency of aspiration following intervention. Unlike the baseline assessment, all participants completed the entire study protocol posttreatment, something they were prohibited from doing due to continued aspiration in the pretreatment condition (aspiration of two consecutive trials stopped participation in the protocol). The study conducted by Juan et al. (2013) reported similar results with a participant 2 years post CVA who exhibited penetration at baseline (PAS = 5) with 10 mL of thin liquids. The participant's posttreatment assessment revealed a PAS range of 2–3 over multiple trials, indicating some improvement, though the participant also used postural strategies and secondary swallows to improve airway protection. All other bolus trials for this participant remained consistent pre- and posttreatment (PAS range = 1–3).
Steele et al. (2013) reported that all six of their participants with closed head injury exhibited aspiration pretreatment (PAS = 7–8) on either thin or spoon-thick liquids. Following treatment, improvement in airway protection was reported in nine out of 12 swallow conditions; however, two participants continued to silently aspirate liquids. In a similar fashion, no change in aspiration frequency was found among participants with oropharyngeal cancer in the treatment arm of the study conducted by Lazarus et al. (2014). The five participants who aspirated during baseline trials continued to do so following intervention.
Oropharyngeal Residue
Changes in oropharyngeal residue, evaluated by rating scales, revealed mixed results. Juan et al. (2013) and Robbins et al. (2007) reported decreased pharyngeal-wall residue following isometric lingual strength training, whereas other oropharyngeal-residue ratings went unchanged. Likewise, Lazarus et al. (2014) completed a t-test comparison of pre/post oropharyngeal swallow-efficiency measures (a combination of transit, residue, and aspiration measures; Rademaker, Pauloski, Logemann, & Shanahan, 1994) and found no significant changes within the experimental group (p = .351).
It should be noted that in some cases, oropharyngeal residue increased following isometric lingual-strengthening exercises. The analyses by Steele et al. (2013) revealed either no change or worsening in rating scales in the residue in the valleculae and pyriform sinuses. Juan et al. (2013) similarly reported increased amounts of residue in the postcricoid space located just above the level of the pharyngoesophageal segment, for a single participant.
Durational Measures
Timing measures, such as bolus clearance in the oral cavity and pharynx, duration of hyoid movement during the swallow, duration and timing of upper esophageal sphincter opening, and total swallowing duration, were evaluated. Significant differences and trends were not consistent across multiple trials of the same presentation for individuals (Juan et al., 2013; Robbins et al., 2007). Overall, there was no evidence of consistent changes in any study (Juan et al., 2013; Lazarus et al., 2014; Robbins et al., 2007).
Lingual Volume
Lingual volume, quantified using calculations derived from MRI, was reported in eight participants across three studies as a percent change of total volume (see Table 6). Researchers reported positive lingual-volume gains in a range of 2%–10%; however, one participant exhibited a −6% decrease (Robbins et al., 2007).
Table 6.
Lingual volumes.
| Study | n | % change |
|---|---|---|
| Juan et al. (2013) | 1 | +8.37 |
| Robbins et al. (2005) | 1 | +10.68 |
| 1 | +2.91 | |
| 1 | +2.16 | |
| 1 | +4.67 | |
| Robbins et al. (2007) | 2 | +4.35 a |
| 1 | −6.5 |
Average of two participants.
Quality-of-Life Measures
Three studies reported standardized quality-of-life measures related to diet textures, emotional outcomes, and psychosocial factors. Juan et al. (2013) reported improvement in 10 out of 11 subscales of the SWAL-QOL (McHorney et al., 2002). Robbins et al. (2007) noted an increased ability for participants to consume regular-texture items following intervention. In the same regard, patients diagnosed with oropharyngeal cancer who received isometric lingual strength training showed significant improvements in the eating domain of the Head and Neck Cancer Inventory (HNCI; Funk, Karnell, Christensen, Moran, and Ricks, 2003), although other domains went unchanged (Lazarus et al., 2014).
Publication Bias: Unpublished Data
Additional unpublished data were provided via personal communication by Steele and colleagues (Appendices B and C; Steele et al., 2007–2010, 2011). Participants in these studies met the same inclusion criteria as described in the work of Steele et al. (2013) and performed the same intervention protocol. However, the number of treatment sessions they received varied. The primary reason these data were excluded from publication was the heterogeneity of the participant diagnoses, which included closed head injury, CVA, brain tumor, and cervical spinal surgery. At baseline, all 17 participants exhibited at least one pressure measure (anterior or posterior) that was under the established typical lingual-pressure range for adults (Adams et al., 2013).
Review of the unpublished maximum peak lingual-pressure changes reflected similar findings as those in published reports, in that isometric lingual-strength intervention improved maximum peak pressure during posttherapy reassessment in populations with dysphagia. Without statistical analysis or effect size values, it is impossible to state whether these gains were significant. However, more than half of participants returned to a typical lingual-pressure range posttherapy (Adams et al., 2013).
In addition to maximum peak lingual-pressure data, the investigators provided lingual-palatal pressure data from saliva swallows for a subset of 16 participants, including data points from the article by Yeates et al. (2008). Once again, a review of the results from unpublished data coincides with those from the four published articles, with varying benefits in carryover to actual swallows for those with dysphagia. One participant increased lingual-pressure generation from 7 to 47 kPa over the course of 24 treatment sessions, whereas other participants maintained values or even decreased. There were no apparent patterns from a simple review of the raw data. Much like the maximum peak lingual pressures, no statistical interpretation was available to assist in determining overall treatment effects.
A full assessment of publication bias was not completed, because the data from the articles in the review were not combined in a meta-analysis. The unpublished data just described reported similar results to those of the peer-reviewed articles, providing additional evidence for the direct benefit of isometric lingual strength training for improved maximum peak pressure gains. Publication bias, or the rejection of articles on the basis of nonfindings, is unlikely to affect the evidence reported in this review article. There would need to be multiple studies with null findings to contradict the evidence reported here.
Analysis of Study Designs: Current Limitations for Meta-Analysis
We initially planned a systematic review with meta-analysis in order to determine and report overall treatment effects. Further review of the design and statistical reporting for each article, unfortunately, determined that they were inappropriate for direct comparison. The studies varied by participant diagnosis, age, treatment length, and pressure targets, all variables with potential influence on lingual-pressure ranges.
Attempts to divide the articles into smaller groupings for analyses yielded additional concerns. Five of the 10 studies enrolled healthy adults; however, descriptive statistical information was reported in only four. Of these, the ages of the participants in the study by Robbins et al. (2005) were not inclusive of the same range as the other three studies (Clark, 2012; Lazarus et al., 2003; Oh, 2015). Although two of the remaining three studies were randomized controlled trials, standard deviations were not always available, making meta-analysis problematic because too few studies could be combined. For the studies that enrolled participants with dysphagia, three used a case-study design with fewer than six participants in each (Juan et al., 2013; Steele et al., 2013; Yeates et al., 2008). Although in general case-study designs provide rich descriptive information on participants, they typically lack the sample sizes needed for statistical analysis and cannot be easily combined with other study designs in meta-analyses. Ideal designs for meta-analysis should provide a control group for comparison purposes. Such comparisons were reported by Clark (2012) and Lazarus et al. (2003, 2014). For easier combination, descriptive statistics or raw data should be reported for all designs.
Discussion
The purpose of this systematic review was to summarize literature on the effects of isometric lingual strength training on lingual strength and swallow function in adults. A high-priority goal within that purpose was to examine the methodological designs of each study, in order to gauge the reported effectiveness of isometric lingual strength training as an intervention for individuals with dysphagia.
A systematic literature search revealed 10 articles for inclusion in the review. From these, two primary lingual-pressure measures were collected: maximum peak lingual pressure and lingual-palatal pressures generated during swallowing. However, not all studies captured swallow data. The articles in this review varied not only by study design (randomized controlled trial, prospective cohort) but also by a number of other factors, including the age and diagnoses of participants, the number of treatment sessions, and the inclusion of swallow parameters reflective of oropharyngeal function. Thus, although a meta-analysis was originally intended, the articles were too heterogeneous for combination.
Four of the studies that included participants with dysphagia reported baseline maximum peak lingual-pressure values that fell below established norms (Juan et al., 2013; Robbins et al., 2007; Steele et al., 2013; Yeates et al., 2008). Of note, three of these articles were qualitative in nature; comparison statistics were not calculated. Rather, the researchers used case-based designs using descriptive statistics. Nevertheless, a simple comparison of raw data revealed positively trending results for maximum peak lingual pressures following intervention. Close examination of the unpublished data also supported the benefits of isometric lingual-strengthening programs to maximum lingual-pressure values in cohorts with dysphagia (Steele et al., 2007–2010, 2011). Studies in which investigators conducted quantitative analyses (p values and Cohen's d) commonly enrolled healthy participants whose baseline lingual-pressure values were within a normal range. Regardless, the healthy participants demonstrated significant gains in maximum peak lingual-pressure values anyway. Thus, gains in maximum peak lingual pressure appear to be consistent across both healthy adults and select populations with dysphagia (Clark, 2012; Clark et al., 2009; Juan et al., 2013; Lazarus et al., 2003; Oh, 2015; Robbins et al., 2005, 2007; Steele et al., 2013; Yeates et al., 2008).
The application of isometric lingual strength training for improved swallow function remains less clear. Less than half of the studies in this review compared changes in lingual-palatal pressure generation during swallows, varying trials by bolus consistency and applied effort (i.e., use of effortful swallow maneuver). Steele et al. (2013) reported that only one out of the six participants in their cohort demonstrated a significant increase in lingual-pressure generation during saliva swallows. Robbins et al. (2007) similarly demonstrated inconsistent findings when analyzing swallow pressures via a series of t-test comparisons. Although two studies reported statistically significant gains, they enrolled healthy participants only (Oh, 2015; Robbins et al., 2005).
The studies by Steele et al. (2013) and Yeates et al. (2008) used a dynamic swallowing model, capturing data during effortful saliva swallows in addition to data collected during isometric strengthening exercises. The authors hypothesized that effortful palatal-pressure contact during swallowing exercises would generalize more readily to bolus swallows than would isometric exercises. However, at baseline, one subject (Participant 5) could not elicit a saliva swallow. Once he completed 10 treatment sessions, he then was able to initiate a swallow and complete treatment as originally planned. In this case, it is possible that isometric lingual strength training created the necessary lingual strength gains by which the participant could then elicit a saliva swallow. Thus, isometric lingual exercise may be of clinical use in those individuals with severe dysphagia, where it could yield benefits in basic swallow function. Whether isometric exercise is then capable of producing further gains beyond general swallow initiation is less clear. It may be that treatment programs were not dosed such that sufficient changes in swallow palatal-pressure generation were achieved. Further research among individuals with dysphagia is needed to determine if isometric lingual exercises later generalize to lingual-palatal pressure generation during swallowing.
In addition to assessing maximum peak and swallow pressures, some of the investigators sought to examine how isometric lingual strength-training programs affected oropharyngeal swallowing parameters, including those pertaining to airway protection, oropharyngeal duration, oropharyngeal residue, tongue volume, and quality of life (SWAL-QOL; HNCI). The outcome data demonstrated a trend toward improved airway protection, as rated with the PAS, which is congruent with the positive changes expressed in quality-of-life measures. For example, participants reported increased ability to consume different textures and upgrade to regular consistencies while decreasing negative emotions surrounding meal times and social situations (Juan et al., 2013; Robbins et al., 2007). Further gains were noted in a small subset of participants in lingual volume, though once again, these results were not consistent for all examined (Juan et al., 2013; Robbins et al., 2005, 2007). Lingual strength-training exercises, interestingly, did not generalize to durational measures or pharyngeal strength, as measured via oropharyngeal-residue scales. Residue scores mostly remained unchanged, but in some cases they worsened after treatment (Juan et al., 2013; Steele et al., 2013). Evidence for the exact association between improved lingual strength and improved pharyngeal strength is lacking. Although the investigations described in this review article linked their outcomes to changes in oropharyngeal swallow function, none mentioned how isometric lingual strength-training tasks might also affect sensory input to the lingual region. Whether the nature of strength-training tasks changes or enhances sensory input is a question for future study.
Evidenced-based practice reviews have been published for various aspects of dysphagia treatment (Ashford et al., 2009; Frymark et al., 2009; McCabe et al., 2009; Wheeler-Hegland, Ashford, et al., 2009; Wheeler-Hegland, Frymark, et al., 2009); however, to our knowledge, a review regarding the best treatment protocol for isometric lingual strength-training programs has not yet been developed. Although this systematic review also sought to complete a meta-analysis to determine treatment effects, the variation across studies did not allow for combination. Studies varied by the status of the participants, along with other known covariates of lingual-pressures measures such as age, palatal target, and bolus viscosity (Adams et al., 2013; Gingrich et al., 2012; Nicosia et al., 2000; Todd et al., 2013). A primary recommendation is to proceed with larger, more homogeneous samples of participants in specific patient populations that have been identified as being at risk for decreased lingual strength. Results could shed light on specific benefits for distinct patient populations and direct intervention for those etiologic groups that are responsive to isometric lingual-strengthening intervention.
For the purposes of determining the overall effectiveness of an intervention, randomized controlled trials are usually considered the highest level of evidence (but see Verdolini Abbott, Barton, Terhorst, & Shembel, 2016). Only three studies presented in this review article evaluated lingual strength training at that rigorous level, and one did not report any significant improvements following treatment (Lazarus et al., 2014). Moving forward, randomized controlled trials across various patient populations would benefit this body of literature and add support for (or potentially refute) our current findings.
Furthermore, exercise protocols used across studies were incongruent in their choice of dosage. In this review, intensity targets were reported to begin as low as 20% of personal maximum peak pressure (Steele et al., 2013) but worked up to 80% in many studies (Juan et al., 2013; Oh, 2015; Robbins et al., 2005, 2007). Likewise, the number of repetitions per task ranged from 10 to 60, and multiple pressure placements were observed throughout exercise designs. Review of the outcome variables (maximum peak lingual pressure, swallow palatal-pressure generation, and select oropharyngeal measures) did not appear to uncover any influence of any of these factors. However, these designs may have not enrolled enough participants to discriminate between these differences with regard to strength and swallow gains.
This review did not examine how strengthening one region of the tongue would generalize to other strength tasks, because few studies examined this phenomenon (Clark, 2012). It is possible that anterior and posterior lingual strength may separately influence the kinematic abilities of the lingual musculature during oral manipulation and propulsion of different bolus consistencies, because anterior lingual strength appears more crucial for lower viscosities and posterior strength for higher (Gingrich et al., 2012; Nicosia et al., 2000; Todd et al., 2013). Moreover, exercise regimens that include tongue lateralization have not received much attention in the literature, and few studies have addressed normative values for these directional tasks (Clark et al., 2009; Clark & Solomon, 2012). The medial position, located between the anterior and posterior palate contacts, is similarly underreported, which Todd et al. (2013) ascribe to differences in the shape of the palate and variability in contact pressure. Future studies need to compare the effects of training tongue regions and whether training medial and lateral targets is beneficial to improving discrete performance of the lingual musculature during swallowing. Determining these effects would allow clinicians to focus on specific lingual exercise tasks and target ranges on the basis of the presentation of oropharyngeal dysphagia deficits.
Clinical Implications
Isometric lingual exercises target an accessible muscle group (the lingual musculature) and are a readily available clinical task for patients. However, there is not enough evidence at this time to confidently report that isometric lingual strength training generalizes to functional swallowing tasks in individuals with dysphagia. Although published results report improvements in commonly used functional outcome measures (i.e., PAS, quality of life), the number of studies is low and their findings variable. Among the published findings are a few questionable outcomes, such as increased pharyngeal residue posttreatment (Juan et al., 2013; Steele et al., 2013). As with any treatment program or strategy that has limited or variable evidence for use, clinicians should use caution when implementing isometric lingual-strengthening exercises as a sole means to improve oropharyngeal swallow function. Clinicians must always monitor their patients' progress for any unintended negative effects of the treatment in use.
This review, importantly, highlights how different patient populations may respond to isometric lingual strengthening as a means for improving lingual strength and the functional swallow. The participants diagnosed with head and neck cancer in the study by Lazarus et al. (2014) did not exhibit any consistent benefits in lingual-strength or swallow outcomes following intervention. Individuals in that study exhibited increased rates of attrition and decreased adherence to the strengthening protocol, which likely affected the treatment outcomes. It is well known in clinical contexts that the complex nature of head and neck cancer and the necessary concomitant treatments required for disease management make adherence to any type of rehabilitation effort difficult for some individuals. Future studies should focus on evaluating treatments with this population while considering the complexity of their diagnoses and the ability of patients to comply with rehabilitation recommendations, relative to the timing, frequency, and duration of the intervention.
Limitations
Though we sought to follow the recommendations set forth in the PRISMA guidelines (Liberati et al., 2009; Moher et al., 2009), this systematic review is not without its limitations. In compliance with current recommendations, three databases, article citations, and supplemental materials (i.e., unpublished data) were searched and compiled for this review article; however, it is possible that the search may not have yielded all available articles of interest. Moving forward, we recommend that the review be updated to reflect the most recent advances in isometric lingual strength-training interventions and potentially be expanded to include additional databases and search terms not attempted in this review article.
Last, two articles had data excluded from the review because they did not report raw values, instead reporting the data in graphical representations. Despite our attempts to contact the authors, pre/post comparison values could not be obtained. These articles would have provided additional information on penetration-aspiration and lingual-pressure generation during swallowing in individuals with dysphagia, two areas that are currently lacking data. This systematic review, therefore, is limited in its ability to report all potential outcome data on the basis of the reporting preferences of authors or journals and the inability to gather further information.
Conclusion
This systematic review described the effects of isometric lingual strength-training programs on lingual strength and swallow function across 10 studies. In particular, the ability for isometric lingual strength training to improve maximum peak lingual pressures in healthy adults and individuals with dysphagia is the most noteworthy finding here. There are limited data to support the direct benefits of isometric lingual strength training to changes in other oropharyngeal swallowing parameters, which may be due to the fact that swallowing requires only submaximal lingual-palatal pressure generation. Determining if isometric lingual strength training generalized to functional swallowing for individuals with dysphagia was not possible in this review. Of clinical importance, the one study that enrolled participants with oropharyngeal cancer did not report gains in lingual strength or measures of oropharyngeal function, which may be due in large part to the nature and complexity of the care required for individuals with this diagnosis. Further research using randomized controlled trial design and different cohorts of participants with dysphagia is required to tease out the benefits of isometric lingual strengthening as it pertains to specific diagnoses. Implications and considerations may arise regarding the timing, intensity, and duration of the intervention program, because some diagnoses may be less responsive to treatment than others.
Acknowledgment
This project was supported by National Center for Advancing Translational Sciences Award UL1TR000077, awarded to The University of Cincinnati. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A
Search Strings
| Database | Search Strings |
|---|---|
| Science Citation Index | TOPIC: (tongue or lingual) AND TOPIC: (strength* and exercise) |
| Science Direct | TITLE-ABSTR-KEY(tongue or lingual) and TITLE-ABSTR-KEY(strength* and exercise). |
| PubMed | (tongue[Title/Abstract] OR lingual[Title/Abstract])) AND (strength*[Title/Abstract] AND exercise[Title/Abstract]) |
Note. A non-language restricted search from 1965 to September 2015 was completed across all three databases.
Appendix B
Pre/Post Measures for Maximum Peak Lingual Pressure: Unpublished Data
| Study | Participant status | Age (years) | Instrument | n | Training days | Bulb replacement | Pre (SD; kPa) | Post (SD; kPa) | Study design and source information |
|---|---|---|---|---|---|---|---|---|---|
| Steele et al. (2007–2010) | CHI | — | IOPI | 13 | 35 | Anterior | 26.7 (4.7) | 33.5 (2.0) | Prospective cohort intervention: Data are part of larger study with the same protocol as described by Steele et al. (2013), varying only in the number of treatment sessions. Inclusion criteria included physiologic measures of premature spillage and/or vallecular residue. |
| Posterior | 23.6 (6.1) | 32.5 (1.7) | |||||||
| CVA | 8 | Anterior | 16.4 (2.0) | 34.3 (1.9) | |||||
| Posterior | 17.1 (3.5) | 32.0 (1.8) | |||||||
| CHI | 14 | Anterior | 31.1 (6.2) | 40.1 (7.6) | |||||
| Posterior | 22.3 (8.4) | 35.0 (5.0) | |||||||
| CVA | 12 | Anterior | 23.8 (3.4) | 37.7 (2.6) | |||||
| Posterior | 25.4 (3.4) | 36.1 (0.9) | |||||||
| Brainstem cavernous hemangioma | 12 | Anterior | 31.4 (1.1) | 33.0 (1.4) | |||||
| Posterior | 29.6 (2.4) | 33.1 (1.5) | |||||||
| Cervical spine injury | 12 | Anterior | 39.5 (4.0) | 56.7 (4.5) | |||||
| Posterior | 50.3 (3.2) | 50.3 (3.1) | |||||||
| CVA | 12 | Anterior | 21.0 (2.3) | 39.6 (3.0) | |||||
| Posterior | 19.7 (4.0) | 35.4 (2.4) | |||||||
| Brainstem tumor | 18 | Anterior | 38.1 (1.7) | 44.7 (0.8) | |||||
| Posterior | 35.3 (2.6) | 39.0 (2.0) | |||||||
| CVA | 10 | Anterior | 61.9 (2.8) | 79.5 (2.9) | |||||
| Posterior | 53.1 (6.7) | 68.9 (3.7) | |||||||
| CVA | 20 | Anterior | 37.8 (1.8) | 47.9 (1.4) | |||||
| Posterior | 35.9 (2.4) | 47.7 (1.5) | |||||||
| CVA | 14 | Anterior | 33.9 (4.2) | 26.8 (2.6) | |||||
| Posterior | 26.9 (8.0) | 23.8 (3.8) | |||||||
| CHI | 39 | Anterior | 35.0 (2.2) | 38.7 (5.7) | |||||
| Posterior | 32.3 (7.1) | 38.4 (4.2) | |||||||
| CHI | 14 | Anterior | 37.9 (8.0) | 40.2 (5.2) | |||||
| Posterior | 31.4 (8.0) | 27.7 (7.4) | |||||||
| Steele et al. (2011) | Skull-base tumor | 62 | IOPI | 4 | 23 | Anterior | 29.2 (2.8) | 50.4 (1.7) | Prospective cohort intervention: Data were presented as an oral presentation at the Dysphagia Research Society. Participants completed the same protocol as reported by Steele et al. (2013). |
| Posterior | 25.3 (4.6) | 44.7 (3.2) | |||||||
| CVA | 66 | 24 | Anterior | 37.9 (3.5) | 46.1 (2.8) | ||||
| Posterior | 24.5 (4.7) | 33.8 (5.3) | |||||||
| CVA | 44 | 24 | Anterior | 53.1 (2.3) | 72.1 (2.3) | ||||
| Posterior | 41.4 (3.3) | 51.7 (1.4) | |||||||
| CVA | 77 | 24 | Anterior | 39.9 (4.2) | 52.5 (2.6) | ||||
| Posterior | 32.6 (3.5) | 56.7 (5.4) |
Note. Exact ages of adult participants in the Steele et al. (2007–2011) data are not known.
CHI = closed head injury; IOPI = Iowa Oral Performance Instrument; CVA = cerebrovascular accident.
Appendix C
Pre/Post Measures for Lingual-Palatal Pressures During Swallows: Unpublished Data
| Study | Participant status | Age (years) | Instrument | n | Training days | Bulb placement | Assessment | Pre (SD; kPa) | Post (SD; kPa) |
|---|---|---|---|---|---|---|---|---|---|
| Steele et al. (2007–2010) | CHI | — | IOPI | 13 | 35 | Anterior | Saliva swallow | 27.7 (4.4) | 17.8 (1.4) |
| CVA | 8 | ||||||||
| CHI | 14 | 9.7 (4.9) | 26.2 (13.3) | ||||||
| CVA | 12 | 14.8 (2.3) | 27.5 (8.3) | ||||||
| Brainstem cavernous hemangioma | 12 | 6.0 (3.7) | 5.0 (0.0) | ||||||
| Cervical spine surgery | 12 | 30.2 (5.7) | |||||||
| CVA | 12 | 19.3 (1.4) | 34.0 (6.9) | ||||||
| Brainstem tumor | 18 | 23.5 (12.7) | 11.2 (5.2) | ||||||
| CVA | 10 | 33.2 (4.4) | 41.0 (10.4) | ||||||
| CVA | 20 | 19.6 (11.8) | 13.2 (6.2) | ||||||
| CVA | 14 | 16.9 (2.9) | 12.3 (5.7) | ||||||
| CHI | 39 | 27.3 (5.9) | |||||||
| CHI | 14 | 24.0 (5.7) | |||||||
| Steele et al. (2011) | Skull-base tumor | 62 | IOPI | 4 | 23 | Anterior | Saliva swallow | 10.3 (1.2) | 18.7 (4.6) |
| CVA | 66 | 24 | 6.8 (1.9) | 11.4 (2.8) | |||||
| CVA | 44 | 24 | 39.7 (5.3) | 46.5 (4.9) | |||||
| CVA | 77 | 24 | 7.2 (2.0) | 47.5 (3.7) | |||||
| Yeates et al. (2008) | CVA | 72 | IOPI | 3 | 8 | Anterior | Saliva swallow | 40.8 (5.1) | 61.9 (7.7) |
| Brain tumor | 63 | 8 | 11.5 (2.1) | 17.3 (2.0) | |||||
| MVA & CVA | 50 | 90 | |||||||
| 138 |
Note. Exact ages of adult participants in the Steele et al. (2007–2011) data are not known.
CHI = closed head injury; IOPI = Iowa Oral Performance Instrument; CVA = cerebrovascular accident (CVA); MVA = motor vehicle accident.
Funding Statement
This project was supported by National Center for Advancing Translational Sciences Award UL1TR000077, awarded to The University of Cincinnati. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
References marked with an asterisk indicate articles included in the systematic review.
- Adams V., Mathisen B., Baines S., Lazarus C., & Callister R. (2013). A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia, 28, 350–369. doi:10.1007/s00455-013-9451-3 [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2009). Progression models in resistance training for healthy adults [Position stand]. Medicine and Science in Sports & Exercise, 41, 687–708. doi:10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- Ashford J., McCabe D., Wheeler-Hegland K., Frymark T., Mullen R., Musson N., … Hammond C. S. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III—Impact of dysphagia treatments on populations with neurological disorders. Journal of Rehabilitation Research & Development, 46, 195–204. doi:10.1682/JRRD.2008.08.0091 [PubMed] [Google Scholar]
- Carlson D. J., Dieberg G., Hess N. C., Millar P. J., & Smart N. A. (2014). Isometric exercise training for blood pressure management: A systematic review and meta-analysis. Mayo Clinic Proceedings, 89, 327–334. doi:10.1016/j.mayocp.2013.10.030 [DOI] [PubMed] [Google Scholar]
- *Clark H. M. (2012). Specificity of training in the lingual musculature. Journal of Speech, Language, and Hearing Research, 55, 657–667. doi:10.1044/1092-4388(2011/11-0045) [DOI] [PubMed] [Google Scholar]
- *Clark H. M., O'Brien K., Calleja A., & Corrie S. N. (2009). Effects of directional exercise on lingual strength. Journal of Speech, Language, and Hearing Research, 52, 1034–1047. doi:10.1044/1092-4388(2009/08-0062) [DOI] [PubMed] [Google Scholar]
- Clark H. M., & Solomon N. P. (2012). Age and sex differences in orofacial strength. Dysphagia, 27, 2–9. doi:10.1007/s00455-011-9328-2 [DOI] [PubMed] [Google Scholar]
- Crow H. C., & Ship J. A. (1996). Tongue strength and endurance in different aged individuals. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 51A, M247–M250. doi:10.1093/gerona/51A.5.M247 [DOI] [PubMed] [Google Scholar]
- Dodds W. J. (1989). The physiology of swallowing. Dysphagia, 3, 171–178. doi:10.1007/BF02407219 [DOI] [PubMed] [Google Scholar]
- Frymark T., Schooling T., Mullen R., Wheeler-Hegland K., Ashford J., McCabe D., … Hammond C. S. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part I—Background and methodology. Journal of Rehabilitation Research & Development, 46, 175–184. doi:10.1682/JRRD.2008.08.0095 [PubMed] [Google Scholar]
- Funk G. F., Karnell L. H., Christensen A. J., Moran P. J., & Ricks J. (2003). Comprehensive head and neck oncology health status assessment. Head & Neck, 25, 561–575. [DOI] [PubMed] [Google Scholar]
- Gingrich L. L., Stierwalt J. A. G., Hageman C. F., & LaPointe L. L. (2012). Lingual propulsive pressures across consistencies generated by the anteromedian and posteromedian tongue by healthy young adults. Journal of Speech, Language, and Hearing Research, 55, 960–972. doi:10.1044/1092-4388(2011/10-0357) [DOI] [PubMed] [Google Scholar]
- Hamanaka-Kondoh S., Kondoh J., Tamine K.-I., Hori K., Fujiwara S., Maeda Y., … Ono T. (2014). Tongue pressure during swallowing is decreased in patients with Duchenne muscular dystrophy. Neuromuscular Disorders, 24, 474–481. [DOI] [PubMed] [Google Scholar]
- Hori K., Ono T., Iwata H., Nokubi T., & Kumakura I. (2005). Tongue pressure against hard palate during swallowing in post-stroke patients. Gerontology, 22, 227–233. [DOI] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., … Buchbinder R. (2012). Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology, 65, 934–939. doi:10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Husaini H., Krisciunas G. P., Langmore S., Mojica J. K., Urken M. L., Jacobson A. S., & Lazarus C. L. (2014). A survey of variables used by speech-language pathologists to assess function and predict functional recovery in oral cancer patients. Dysphagia, 29, 376–386. doi:10.1007/s00455-014-9520-2 [DOI] [PubMed] [Google Scholar]
- *Juan J., Hind J., Jones C., McCullough T., Gangnon R., & Robbins J. (2013). Case study: Application of isometric progressive resistance oropharyngeal therapy using the Madison Oral Strengthening Therapeutic device. Topics in Stroke Rehabilitation, 20, 450–470. doi:10.1310/tsr2005-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays S., & Robbins J. (2006). Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Seminars in Speech and Language, 27, 245–259. doi:10.1055/s-2006-955115 [DOI] [PubMed] [Google Scholar]
- Kier W. M., & Smith K. K. (1985). Tongues, tentacles and trunks: The biomechanics of movement in muscular-hydrostats. Zoological Journal of the Linnean Society, 83, 307–324. [Google Scholar]
- Konaka K., Kondo J., Hirota N., Tamine K., Hori K., Ono T., … Naritomi H. (2010). Relationship between tongue pressure and dysphagia in stroke patients. European Neurology, 64, 101–107. doi:10.1159/000315140 [DOI] [PubMed] [Google Scholar]
- Lazarus C. (2006). Tongue strength and exercise in healthy individuals and in head and neck cancer patients. Seminars in Speech and Language, 27, 260–267. [DOI] [PubMed] [Google Scholar]
- *Lazarus C. L., Husaini H., Falciglia D., DeLacure M., Branksi R. C., Kraus D., … Sanfilippo N. (2014). Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. International Journal of Oral & Maxillofacial Surgery, 43, 523–530. doi:10.1016/j.ijom.2013.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lazarus C., Logemann J. A., Huang C.-F., & Rademaker A. W. (2003). Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica et Logopaedica, 55, 199–205. doi:10.1159/000071019 [DOI] [PubMed] [Google Scholar]
- Lazarus C. L., Logemann J. A., Pauloski B. R., Rademaker A. W., Larson C. R., Mittal B. B., … Pierce M. (2000). Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of Speech, Language, and Hearing Research, 43, 1011–1023. doi:10.1044/jslhr.4304.1011 [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A., … Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. The BMJ, 339, b2700 doi:10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki G. A., Kaufman A., Hind J., Ennis S., Gangnon R., Waclawik A., & Robbins J. (2012). The effects of lingual intervention in a patient with inclusion body myositis and Sjögren's syndrome: A longitudinal case study. Archives of Physical Medicine and Rehabilitation, 93, 1469–1475. doi:10.1016/j.apmr.2012.02.010 [DOI] [PubMed] [Google Scholar]
- McCabe D., Ashford J., Wheeler-Hegland K., Frymark T., Mullen R., Musson N., … Schooling T. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part IV—Impact of dysphagia treatment on individuals' postcancer treatments. Journal of Rehabilitation Research & Development, 46, 205–214. doi:10.1682/JRRD.2008.08.0092 [PubMed] [Google Scholar]
- McHorney C. A., Robbins J., Lomax K., Rosenbek J. C., Chignell K., Kramer A. E., & Bricker D. E. (2002). The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia, 17, 97–114. [DOI] [PubMed] [Google Scholar]
- Miller J. L., & Watkin K. L. (1996). The influence of bolus volume and viscosity on anterior lingual force during the oral stage of swallowing. Dysphagia, 11, 117–124. [DOI] [PubMed] [Google Scholar]
- Miller J. L., Watkin K. L., & Chen M. F. (2002). Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. Journal of Speech, Language, and Hearing Research, 45, 51–65. doi:10.1044/1092-4388(2002/004) [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., & the PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia M. A., Hind J. A., Roecker E. B., Carnes M., Doyle J., Dengel G. A., & Robbins J. (2000). Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 55, M634–M640. doi:10.1093/gerona/55.11.M634 [DOI] [PubMed] [Google Scholar]
- *Oh J.-C. (2015). Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia, 30, 315–320. doi:10.1007/s00455-015-9601-x [DOI] [PubMed] [Google Scholar]
- Palmer P. M., Neel A. T., Sprouls G., & Morrison L. (2010). Swallow characteristics in patients with oculopharyngeal muscular dystrophy. Journal of Speech, Language, and Hearing Research, 53, 1567–1578. doi:10.1044/1092-4388(2010/09-0068) [DOI] [PubMed] [Google Scholar]
- Rademaker A. W., Pauloski B. R., Logemann J. A., & Shanahan T. K. (1994). Oropharyngeal swallow efficiency as a representative measure of swallowing function. Journal of Speech and Hearing Research, 37, 314–325. doi:10.1044/jshr.3702.314 [DOI] [PubMed] [Google Scholar]
- *Robbins J., Gangnon R. E., Theis S. M., Kays S. A., Hewitt A. L., & Hind J. A. (2005). The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society, 53, 1483–1489. doi:10.1111/j.1532-5415.2005.53467.x [DOI] [PubMed] [Google Scholar]
- *Robbins J., Kays S. A., Gangnon R. E., Hind J. A., Hewitt A. L., Gentry L. R., & Taylor A. J. (2007). The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation, 88, 150–158. doi:10.1016/j.apmr.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Rosenbek J. C., Robbins J., Roecker E. B., Coyle J. L., & Woods J. L. (1996). A penetration-aspiration scale. Dysphagia, 11, 93–98. doi:10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- Stål P., Marklund S., Thornell L.-E., De Paul R., & Eriksson P.-O. (2003). Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs, 173, 147–161. doi:10.1159/000069470 [DOI] [PubMed] [Google Scholar]
- *Steele C. M., Bailey G. L., Polacco R. E. C., Hori S. F., Molfenter S. M., Oshalla M., & Yeates E. M. (2013). Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. International Journal of Speech-Language Pathology, 15, 492–502. doi:10.3109/17549507.2012.752864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Bressmann T., & Carnahan H. (2007–2010). [The role of skilled tongue pressure generation in swallowing]. Unpublished raw data. [Google Scholar]
- Steele C., Molfenter S., Bailey G., Oshalla M., & Yeates E. (2011). Tongue-pressure strength and accuracy training (TPSAT) for thin liquid dysphagia [Abstract]. Dysphagia, 26, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. T., Lintzenich C. R., & Butler S. G. (2013). Isometric and swallowing tongue strength in healthy adults. The Laryngoscope, 123, 2469–2473. doi:10.1002/lary.23852 [DOI] [PubMed] [Google Scholar]
- Unemoto G., Tsuboi Y., Kitashima A., Furuya H., & Kikuta T. (2011). Impaired food transportation in Parkinson's disease related to lingual bradykinesia. Dysphagia, 26, 250–255. doi:10.1007/s00455-010-9296-y [DOI] [PubMed] [Google Scholar]
- Vanderwegen J., Guns C., Van Nuffelen G., Elen R., & De Bodt M. (2013). The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia, 28, 159–166. doi:10.1007/s00455-012-9425-x [DOI] [PubMed] [Google Scholar]
- Verdolini Abbott K., Barton F. B., Terhorst L., & Shembel A. (2016). Retrospective studies: A fresh look. American Journal of Speech-Language Pathology, 25, 157–163. doi:10.1044/2016_AJSLP-16-0025 [DOI] [PubMed] [Google Scholar]
- Wheeler-Hegland K., Ashford J., Frymark T., McCabe D., Mullen R., Musson N., … Schooling T. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part II—Impact of dysphagia treatment on normal swallow function. Journal of Rehabilitation Research & Development, 46, 185–194. doi:10.1682/JRRD.2008.08.0094 [PubMed] [Google Scholar]
- Wheeler-Hegland K., Frymark T., Schooling T., McCabe D., Ashford J., Mullen R., … Musson N. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part V—Applications for clinicians and researchers. Journal of Rehabilitation Research & Development, 46, 215–222. doi:10.1682/JRRD.2008.08.0093 [PubMed] [Google Scholar]
- *Yeates E. M., Molfenter S. M., & Steele C. M. (2008). Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: Three case reports. Clinical Interventions in Aging, 3, 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans S. R., & Stierwalt J. A. G. (2006). Measures of tongue function related to normal swallowing. Dysphagia, 21, 102–111. doi:10.1007/s00455-006-9013-z [DOI] [PubMed] [Google Scholar]
- Youmans S. R., Youmans G. L., & Stierwalt J. A. G. (2009). Differences in tongue strength across age and gender: Is there a diminished strength reserve? Dysphagia, 24, 57–65. doi:10.1007/s00455-008-9171-2 [DOI] [PubMed] [Google Scholar]