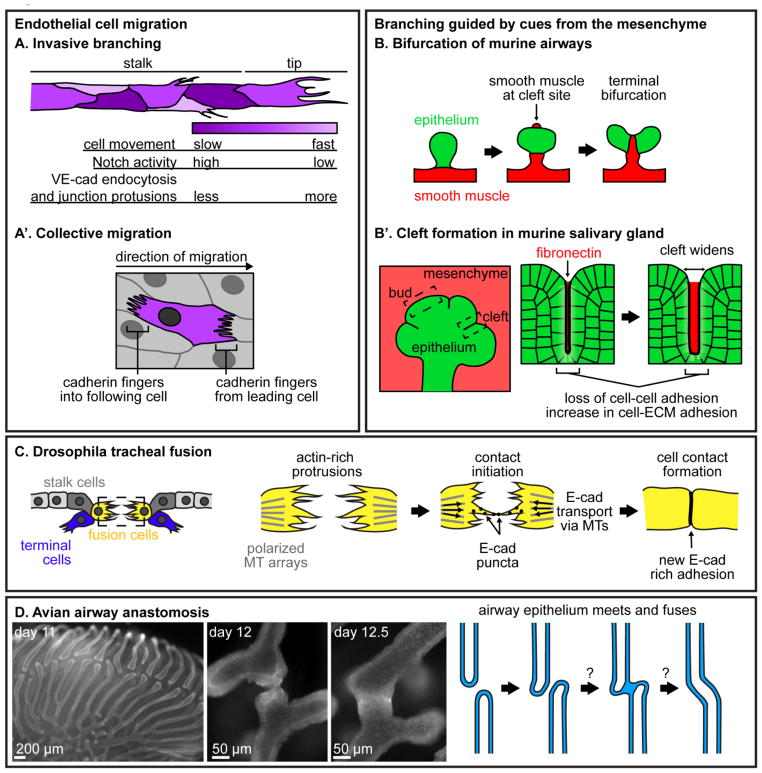
Figure 2. Branching and tube fusion.
(A-A′) Migration of endothelial cells in different contexts. (A) During invasive branching, endothelial cells at the tip of a blood vessel exhibit different levels of Notch signaling, endocytose VE-cadherin at different rates, and shuffle either more quickly or more slowly. As a result of this dynamic behavior, tip-cell identity switches between neighbors as the vessel migrates. (A′) Endothelial cells migrating collectively engulf cadherin fingers from cells ahead of them and extend cadherin fingers into the cells following them. (B-B′) Examples of branching guided by cues from the surrounding mesenchyme. (B) Bifurcation of the murine airway epithelium is guided by smooth muscle differentiation. As the parent branch grows, smooth muscle appears at the future cleft site. Smooth muscle wraps around the epithelium in a specific pattern, dividing it in two and leading to bifurcation. (B′) During branching morphogenesis of the murine salivary gland, fibronectin accumulates in clefts, leading to an increase in cell-ECM adhesion and a breakdown of cell-cell adhesion to allow clefts to widen. (C) During tracheal fusion in the Drosophila embryo, fusion cells extend filopodia towards each other. When filopodia meet, E-cadherin puncta move along filopodia to initiate a new adhesion, and E-cadherin is trafficked along microtubules (MTs) towards the new cell-cell junction. (D) Anastomosis of parabronchi during avian airway morphogenesis has not yet been extensively studied. Multicellular parabronchi extend towards each other and fuse by unknown mechanisms to generate loops required for unidirectional air flow. Images of E-cadherin immunofluorescence in lungs from embryonic days 11, 12 and 12.5 show the different stages of airway anastomosis.