Abstract
To study the role of the absolute lymphocyte count (ALC), absolute monocyte count (AMC), platelet count (PLT), lymphocyte–monocyte ratio (LMR) and the platelet–lymphocyte ratio (PLR) in the prognosis of primary gastrointestinal diffuse large B cell lymphoma (PGI-DLBCL). We retrospectively analyzed the prognostic value of the ALC, AMC, PLT, LMR, PLR at diagnosis in 173 PGI-DLBCL patients through histopathological examination from March 2009 to February 2015. In total, 173 patients with histopathological diagnosis of PGI-DLBCL in this study, the median age was 51 years (range 12–90 years), median follow-up time was 44 months (range 7–89 months). In univariate analysis, age <60 years, B symptoms, Lugano stage I–II, low international prognostic index (IPI) or low age-adjusted international prognostic index (aaIPI), normal lactate dehydrogenase (LDH), normal β2-microglobulin (β2m), Hb ≥ 11 g/dL, ALC ≥ 1.5 × 109/L, AMC ≤ 0.50 × 109/L, LMR ≥ 2.5, PLR ≤ 170 were related with superior overall survival (OS) and progression-free survival (PFS) (p ≤ 0.05). Multivariate analysis suggested that ALC, LMR, LDH were related with PFS (p ≤ 0.05). Similarly, age and LMR were related with OS (p ≤ 0.05). The parameters (ALC, AMC, LMR, PLR) may be valuable prognostic factors in PGI-DLBCL patients. LMR, PLR at diagnosis are expected to be independent prognostic factors for PGI-DLBCL patients.
Keywords: Primary gastrointestinal diffuse large B-cell lymphoma, The lymphocyte–monocyte ratio, The platelet–lymphocyte ratio, Inflammatory, Prognosis
Introduction
Primary gastrointestinal non Hodgkin’s lymphoma (PGI-NHL) represents the most common types of extranodal lymphoma and DLBCL is the most frequent histologic type. PGI-DLBCL is a relatively rare disease, accounting for only 1–4 % of those with gastrointestinal malignancies. The most frequent site is stomach followed by small intestine and ileocecal region [1–3]. Endoscopic examination combined with pathological biopsy is still the most important and reliable method for detection of PGI-DLBCL. Nowadays, there is still no consensus on its treatment, the majority of patients were treated with combined therapy (chemotherapy and radiotherapy, surgery plus chemotherapy, surgery plus chemotherapy, and radiotherapy).The majority of chemotherapeutic protocols was CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone)-like regimens and/without rituximab [4–6]. Immunodeficiency is considered to be one of the most dangerous factors in NHL, with a greater risk of developing lymphoma with congenital or acquired immunodeficiency. Gene expression profiling (GEP) have provided crucial information as to the intrinsic heterogeneity of lymphoma cells and the role of the tumor microenvironment. The prognostic model based on gene expression in DLBCL indicates that the prognosis of patients not only dependent on the clinical parameters, but also on the gene regulation of tumor microenvironment [7, 8]. However, many of these risk factors are costly, difficult to perform and not easily interpreted. Therefore, prognostic models for PGI-DLBCL that are inexpensive, widely available and easily interpreted by clinicians are needed [9]. Lymphocyte is the main component of immune cells, which has important anti-tumor effect. It reflects both the immune status of the host and the degree of tumor progression. The systemic inflammation has been recognized as correlating with tumor progression and inflammatory markers have been reported to be useful for predicting the tumor prognosis. The monocytes and platelet are inflammatory markers [4, 10, 11]. Previous studies [7–11] showed that ALC, AMC, PLT, LMR and PLR were prognostic factors for many malignancies. In another study, we found that the LMR could be an efficient prognostic factor for these newly-diagnosed MM patients with extramedullary invasion. However, there are limited data by now to demonstrate their usefulness for PGI-DLBCL. The aim of this study was to determine whether ALC, AMC, PLT, LMR and PLR were prognostic factors for PGI-DLBCL.
Patients and Methods
Patients
We carried out a retrospective study of 173 patients with PGI-DCBCL diagnosed at Tianjin Medical University Cancer Institute and Hospital in March 2009–February 2015. All patients met the following criteria: no previous treatment, no previous history of malignancy, or immunosuppression. There were 111 males and 62 females. The median age was 51 years (range 12–90 years), median follow-up time was 44 months (range 7–89 months). Clinical parameters included patient age, gender, primary site, GCB or nGCB, systemic B symptoms, Lugano stage, IPI, Hb, ALC, AMC, PLT, LDH, β2m, surgery or not. Definitions of response criteria, overall survival, progression-free survival, and time to progression were based on the guidelines from the International Harmonization Project on Lymphoma. OS was defined as the time from diagnosis of PGI-DLBCL to death from any cause. PFS was defined as time from diagnosis to lymphoma relapse, progression or death of any cause.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (Version 17.0). The time-dependent receiver operating characteristic (ROC) curve analysis was used to determine the best cut-off value of preoperative ALC, AMC, LMR, PLR. Survival was analyzed by the Kaplan–Meier and compared by different groups using the log-rank test and the Cox proportional hazards model. p < 0.05 was considered statistically significant.
Results
Selection of the Best Cut-Off Value of ALC, AMC, LMR, PLR for PGI-DCBCL Patients
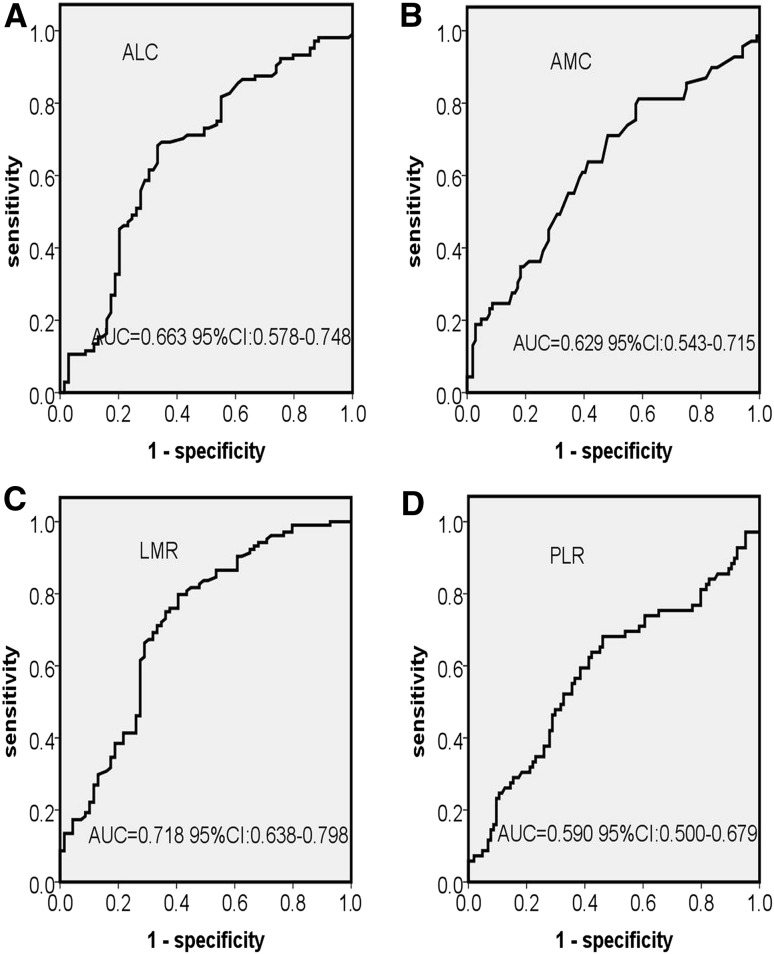
The cutoff points of ALC, AMC, LMR and PLR were selected by the receiver operating characteristics curves analysis. The best cutoff value of ALC was 1.5 × 109/L, with an area under the curve (AUC) value of 0.663 (95 % CI 0.578–0.748, p < 0.001) (Fig. 1a). The most discriminative cutoff value of AMC was 0.5 × 109/L, with an AUC value of 0.629 (95 % CI 0.543–0.715, p = 0.004) (Fig. 1b). ROC curve analysis in 173 patients established 2.5 as the cutoff point of LMR with an AUC of 0.718 (95 % CI 0.638–0.799, p < 0.001) (Fig. 1c) and established 170 as the cutoff point of PLR with an AUC of 0.590 (95 % CI 0.500–0.679, p = 0.004) (Fig. 1d).
Fig. 1.
Receiver operating characteristic curve (ROC) and area under the curve (AUC) for the ALC (a), AMC (b), LMR (c) and PLR (d) at diagnosis. Abbreviations: ALC absolute lymphocyte count, AMC absolute monocyte count, LMR absolute lymphocyte count/absolute monocyte count ratio, PLR platelet count/lymphocyte count ratio, CI confidence interval
In Univariate Analysis: Prognostic Significance of Clinical Parameters
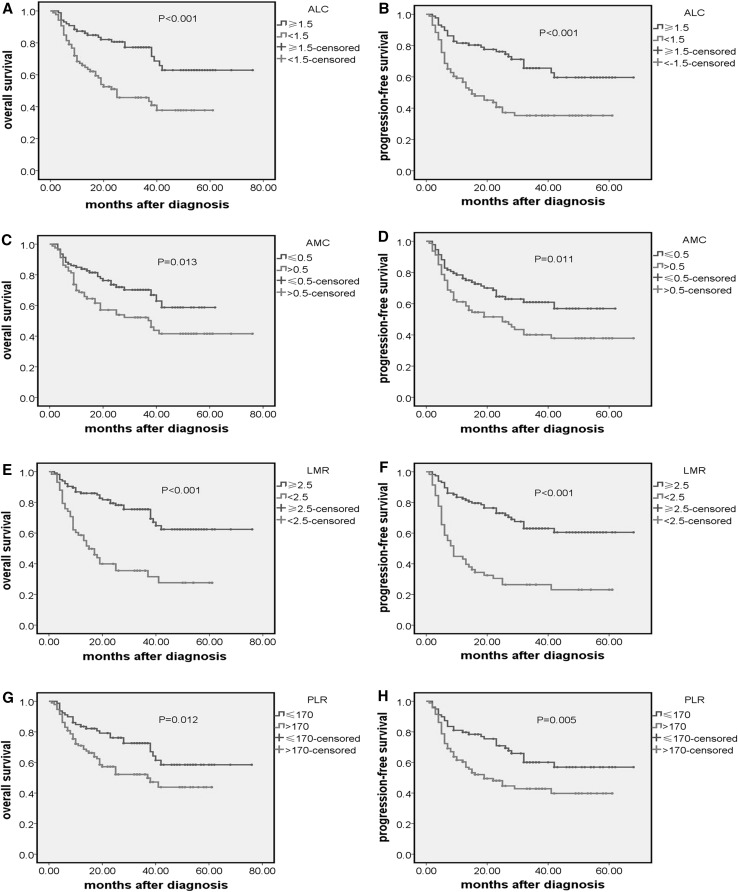
We retrospectively analyzed the prognostic significance of all parameters in 173 patients. We found that patients with an ALC < 1.5 × 109/L had a significantly lower 5-year OS (46.5 vs 73.5 %, p < 0.001) (Fig. 2a) and PFS (39.5 vs 67.8 %, p < 0.001) (Fig. 2b). Patients with an AMC > 0.5 × 109/L had a significantly lower 5-year OS (48.8 vs 69.9 %, p = 0.013) (Fig. 2c) and PFS (42.5 vs 63.4 %, p = 0.011) (Fig. 2d). The estimated 5-year OS and PFS in the patients with LMR < 2.5 and LMR ≥ 2.5 were 34.5 versus 73.0 % (p < 0.001) (Fig. 2e) and 25.9 versus 67.8 % (p < 0.001) (Fig. 2f). The estimated 5-year OS and PFS in the patients with PLR > 170 and PLR ≤ 170 were 52.7 versus 68.8 % (p = 0.012) (Fig. 2g) and 45.7 versus 63.3 % (p = 0.005) (Fig. 2h). On univariate analysis, both the OS and PFS outcomes were associated with age >60 years, systemic B symptoms, stage III–IV, Hb ≤ 11 g/dL, IPI > 2 score, high LDH, high β2m, no surgery. However, gender, primary site, PLT and immune subtype are in no way associated with the OS and PFS outcome (Table 1).
Fig. 2.
Kaplan–Meier curve of overall survival and PFS according to: a, b ALC; c, d AMC; e, f LMR; g, h PLR
Table 1.
In univariate analysis: Prognostic Significance of clinical parameters
| Characteristics | Classification | N = 173 | 5OS (%) | p value | 5PFS (%) | p value |
|---|---|---|---|---|---|---|
| Sex | Male | 111 | 56.8 | 0.128 | 51.4 | 0.300 |
| Female | 62 | 66.1 | 58.1 | |||
| Age | <60 years | 105 | 68.9 | <0.001 | 60.0 | 0.005 |
| ≥60 years | 68 | 47.1 | 44.1 | |||
| Primary site | Stomach | 101 | 64.4 | 0.437 | 58.4 | 0.351 |
| Ileocecal | 16 | 62.5 | 56.3 | |||
| Small intestine | 33 | 57.6 | 48.5 | |||
| Colorectal | 23 | 43.5 | 9.1 | |||
| B symptoms | No | 103 | 65.0 | 0.040 | 60.2 | 0.011 |
| Yes | 70 | 52.9 | 44.3 | |||
| Lugano stage | I–II stage | 110 | 71.8 | <0.001 | 64.5 | <0.001 |
| III–IV stage | 63 | 39.7 | 34.9 | |||
| Immune subtype | GCB | 64 | 65.6 | 0.452 | 62.5 | 0.107 |
| nGCB | 109 | 56.9 | 48.6 | |||
| IPI | ≤2 score | 124 | 73.4 | <0.001 | 66.9 | <0.001 |
| >2 score | 49 | 26.5 | 20.4 | |||
| Hb | >11 g/dL | 118 | 66.9 | 0.009 | 61.0 | 0.004 |
| ≤11 g/dL | 55 | 45.5 | 38.2 | |||
| ALC | ≥1.5 × 109/L | 87 | 73.6 | <0.001 | 67.8 | <0.001 |
| <1.5 × 109/L | 86 | 46.5 | 39.5 | |||
| AMC | ≤0.5 × 109/L | 93 | 69.9 | 0.013 | 63.4 | 0.011 |
| >0.5 × 109/L | 80 | 48.8 | 42.5 | |||
| PLT | ≤250 × 109/L | 71 | 56.3 | 0.323 | 50.7 | 0.353 |
| >250 × 109/L | 102 | 62.7 | 55.9 | |||
| LMR | ≥2.5 | 115 | 73.0 | <0.001 | 67.8 | <0.001 |
| <2.5 | 58 | 34.5 | 25.9 | |||
| PLR | ≤170 | 80 | 68.8 | 0.011 | 68.4 | 0.008 |
| >170 | 93 | 52.7 | 43.2 | |||
| LDH | ≤240 | 107 | 72.0 | <0.001 | 68.2 | <0.001 |
| >240 | 66 | 40.9 | 30.3 | |||
| β2m | ≤2.6 | 97 | 77.3 | <0.001 | 71.1 | <0.001 |
| >2.6 | 76 | 38.2 | 31.6 | |||
| Surgery | No | 58 | 51.7 | 0.012 | 44.8 | 0.008 |
| Yes | 115 | 64.3 | 58.3 |
IPI international prognostic index, Hb hemoglobin, ALC the absolute lymphocyte count, AMC absolute monocyte count, PLT platelet count, LMR lymphocyte–monocyte ratio, PLR platelet–lymphocyte ratio, LDH lactate dehydrogenase, β2m β 2-microglobulin, OS overall survival, PFS progression-free survival
Relationship Between ALC, AMC, LMR, PLR and Other Prognostic Factors
The clinical features of the patients with an ALC ≥ 1.5 × 109/L and those with an ALC < 1.5 × 109/L are summarized in Table 2. Eighty-seven patients (50.3 %) had an ALC ≥ 1.5 × 109/L and 86 patients (49.7 %) had an ALC < 1.5 × 109/L. ALC was significantly correlated with the presence of B symptoms, IPI > 2, Hb ≤ 11 g/dL, a higher LDH (p < 0.05). But not significantly associated with age, primary site, sex, Lugano stage, immune subtype, β2m, surgery (p > 0.05). Ninety-three patients (53.76 %) had an AMC ≤ 0.5 × 109/L and 80 patients (46.24 %) had an AMC > 0.5 × 109/L. We found a significantly difference between the patients with AMC ≤ 0.5 × 109/L and > 0.5 × 109/L regarding male, IPI, β2m (p < 0.05), but not regarding age, primary site, sex, B symptoms, Lugano stage, immune subtype, LDH, Hb, surgery (p > 0.05). The clinical features of the patients with an LMR ≥ 2.5 and those with an LMR < 2.5 are summarized in Table 3. One hundred and fifteen patients (66.7 %) had an LMR ≥ 2.5 and 58 patients (33.53 %) had an LMR < 2.5. LMR was significantly correlated with the presence of B symptoms, a higher β2m, a higher LDH (p < 0.05). But not significantly associated with age, primary site, sex, Lugano stage, immune subtype, Hb, β2m, surgery (p > 0.05). The clinical features of the patients with an PLR ≤ 170 and those with an PLR > 170 are summarized in Table 4. Seventy-nine patients (45.7 %) had an PLR ≤ 170 and 94 patients (54.3 %) had an PLR > 170. We found a significantly difference between the patients with PLR ≤ 170 and >170 regarding the presence of B symptoms, IPI, Lugano stage, Hb ≤ 11 g/dL, β2m, LDH (p < 0.05), but not significantly related with age, primary site, sex, immune subtype, surgery (p > 0.05).
Table 2.
Patient characteristics according to the ALC ≥ 1.5 × 109/L versus <1.5 × 109/L
| Characteristics | Classification | ALC ≥ 1.5 × 109/L | ALC < 1.5 × 109/L | p value |
|---|---|---|---|---|
| Sex | Male | 51 (58.6 %) | 60 (69.8 %) | 0.154 |
| Female | 36 (41.4 %) | 26 (30.2 %) | ||
| Age | <60 years | 55 (63.2 %) | 50 (58.1 %) | 0.535 |
| ≥60 years | 32 (36.8 %) | 36 (41.9 %) | ||
| Primary | Stomach | 55 (63.2 %) | 46 (53.5 %) | 0.405 |
| Ileoceca | 6 (6.9%) | 10 (11.6 %) | ||
| Small intestine | 17 (19.5 %) | 16 (18.6 %) | ||
| Colorectal | 9 (10.3 %) | 14 (16.3 %) | ||
| B symptoms | No | 60 (69.0 %) | 43 (50.0 %) | 0.013 |
| Yes | 27 (31.0 %) | 43 (50.0 %) | ||
| Lugano stage | I–II stage | 59 (67.8 %) | 51 (59.3 %) | 0.271 |
| III–IV stage | 28 (32.2 %) | 35 (40.7 %) | ||
| IPI | ≤2 score | 69 (79.3 %) | 55 (64.0 %) | 0.029 |
| >2 score | 18 (20.7 %) | 31 (36.0 %) | ||
| Immune subtype | GCB | 32 (36.8 %) | 32 (37.2 %) | 1.000 |
| nGCB | 55 (63.2 %) | 54 (62.8 %) | ||
| Hb | >11 g/dL | 69 (79.3 %) | 49 (57.0 %) | 0.002 |
| ≤11 g/dL | 18 (20.7 %) | 37 (43.0 %) | ||
| LDH | ≤240 | 64 (73.6 %) | 43 (50.0 %) | 0.002 |
| >240 | 23 (26.4 %) | 43 (50.0 %) | ||
| β2m | ≤2.6 | 55 (63.2 %) | 42 (48.8 %) | 0.067 |
| >2.6 | 32 (36.8 %) | 44 (51.2 %) | ||
| Surgery | No | 28 (32.2 %) | 30 (34.9 %) | 0.749 |
| Yes | 59 (67.8 %) | 56 (65.1 %) |
IPI international prognostic index, Hb hemoglobin, ALC the absolute lymphocyte count, LDH lactate dehydrogenase, β2m β 2-microglobulin
Table 3.
Patient characteristics according to the LMR ≥ 2.5 versus <2.5
| Characteristics | Classification | LMR ≥ 2.5 | LMR < 2.5 | p value |
|---|---|---|---|---|
| Sex | Male | 67 (58.3 %) | 44 (75.9 %) | 0.065 |
| Female | 48 (41.7 %) | 14 (24.1 %) | ||
| Age | <60 years | 69 (60.0 %) | 36 (62.1 %) | 0.711 |
| ≥60 years | 46 (40.0 %) | 22 (37.9 %) | ||
| Primary site | Stomach | 77 (67.0 %) | 24 (41.4 %) | 0.064 |
| Ileocecal | 8 (7.0 %) | 8 (13.8 %) | ||
| Small intestine | 18 (15.7 %) | 15 (25.9 %) | ||
| Colorectal | 12 (10.4 %) | 11 (18.9 %) | ||
| B symptoms | No | 78 (67.8 %) | 25 (43.1 %) | 0.005 |
| Yes | 37 (32.2 %) | 33 (56.9 %) | ||
| Lugano stage | I–II stage | 78 (67.8 %) | 32 (55.2 %) | 0.135 |
| III–IV stage | 37 (32.2 %) | 26 (44.8 %) | ||
| IPI | ≤2 score | 88 (76.5 %) | 36 (62.1 %) | 0.052 |
| >2 score | 27 (23.5 %) | 22 (37.9 %) | ||
| Immune subtype | GCB | 43 (37.4 %) | 21 (36.2 %) | 0.742 |
| nGCB | 72 (62.6 %) | 37 (63.8 %) | ||
| Hb | >11 g/dL | 83 (72.2 %) | 35 (63.8 %) | 0.199 |
| ≤11 g/dL | 32 (27.8 %) | 23 (39.7 %) | ||
| LDH | ≤240 | 81 (70.4 %) | 26 (44.8 %) | 0.003 |
| >240 | 34 (29.6 %) | 32 (55.2 %) | ||
| β2m | ≤2.6 | 78 (67.8 %) | 19 (32.8 %) | <0.001 |
| >2.6 | 37 (32.2 %) | 39 (67.2 %) | ||
| Surgery | No | 34 (29.6 %) | 24 (41.4 %) | 0.202 |
| Yes | 81 (70.4 %) | 34 (58.6 %) |
IPI international prognostic index, Hb hemoglobin, LMR lymphocyte–monocyte ratio, LDH lactate dehydrogenase, β2m β 2-microglobulin
Table 4.
Patient characteristics according to the PLR ≤ 170 versus >170
| Characteristics | Classification | PLR ≤ 170 | PLR > 170 | p value |
|---|---|---|---|---|
| Sex | Male | 45 (57.0 %) | 66 (70.2 %) | 0.081 |
| Female | 34 (43.0 %) | 28 (29.8 %) | ||
| Age | <60 years | 44 (55.7 %) | 61 (64.9 %) | 0.274 |
| ≥60 years | 35 (44.3 %) | 33 (35.1 %) | ||
| Primary site | Stomach | 44 (67.1 %) | 48 (51.1 %) | 0.189 |
| Ileocecal | 5 (6.3 %) | 11 (11.7 %) | ||
| Small intestine | 12 (15.2 %) | 21 (22.3 %) | ||
| Colorectal | 9 (11.4 %) | 14 (14.9 %) | ||
| B symptoms | No | 55 (69.6 %) | 48 (51.1 %) | 0.019 |
| Yes | 24 (30.4 %) | 46 (48.9 %) | ||
| Lugano stage | I–II stage | 56 (70.9 %) | 54 (57.4 %) | 0.081 |
| III–IV stage | 23 (29.1 %) | 40 (42.6 %) | ||
| IPI | ≤2 score | 64 (81.0 %) | 60 (63.8 %) | 0.017 |
| >2 score | 15 (19.0 %) | 34 (36.2 %) | ||
| Immune subtype | GCB | 27 (34.2 %) | 37 (39.4 %) | 0.529 |
| nGCB | 52 (65.8 %) | 57 (60.6 %) | ||
| Hb | >11 g/dL | 65 (82.3 %) | 53 (56.4 %) | <0.001 |
| ≤11 g/dL | 14 (17.7 %) | 41 (43.6 %) | ||
| LDH | ≤240 | 56 (70.9 %) | 51 (54.3 %) | 0.028 |
| >240 | 23 (29.1 %) | 43 (45.7 %) | ||
| β2m | ≤2.6 | 52 (65.8 %) | 45 (47.9 %) | 0.021 |
| >2.6 | 27 (34.2 %) | 49 (52.1 %) | ||
| Surgery | No | 26 (32.9 %) | 32 (34.0 %) | 1.000 |
| Yes | 53 (67.1 %) | 62 (66.0 %) |
IPI international prognostic index, Hb hemoglobin, PLR platelet–lymphocyte ratio, LDH lactate dehydrogenase, β2m β 2-microglobulin
Multivariate Analysis for OS and PFS Outcomes
We used Cox Regression analysis to evaluate the prognostic impact of LMR at diagnosis on the survival of PGI-DLBCL patients. Parameters included in the multivariate survival analysis are shown in Table 5. This study showed that LMR was identified as an independent prognostic factor for OS and PFS. Age was shown to be independent prognostic factors for OS, while LDH, ALC were independent risk factors for PFS.
Table 5.
Multivariate analysis of prognostic factors for survival in testing set
| Characteristics | p value (OS) | HR (95 % CI) | p value (PFS) | HR (95 % CI) |
|---|---|---|---|---|
| Age | 0.014 | 2.011 (1.154–3.513) | 0.162 | 1.446 (0.861–2.436) |
| B symptoms | 0.856 | 1.047 (0.633–1.735) | 0.404 | 1.211 (0.771–1.905) |
| Lugano stage | 0.287 | 1.461 (0.727–2.937) | 0.344 | 1.352 (0.723–2.532) |
| IPI | 0.085 | 2.027 (0.908–4.521) | 0.134 | 1.764 (0.840–3.702) |
| Hb | 0.800 | 1.075 (0.616–1.876) | 0.414 | 1.243 (0.739–2.088) |
| ALC | 0.079 | 1.869 (0.930–3.748) | 0.024 | 2.145 (1.107–4.135) |
| AMC | 0.529 | 1.207 (0.673–2.163) | 0.310 | 1.329 (0.769–2.288) |
| LMR | 0.043 | 1.636 (0.244–0.976) | 0.009 | 2.014 (0.226–0.813) |
| PLR | 0.461 | 0.763 (0.376–1.557) | 0.150 | 0.590 (0.292–1.207) |
| LDH | 0.109 | 0.488 (0.896–2.992) | 0.011 | 0.428 (1.172–3.471) |
| β2m | 0.075 | 1.694 (0.949–3.026) | 0.170 | 1.464 (0.849–2.527) |
| Surgery | 0.613 | 0.870 (0.508–1.491) | 0.440 | 0.820 (0.497–1.355) |
IPI international prognostic index, Hb hemoglobin, ALC the absolute lymphocyte count, AMC absolute monocyte count, LMR lymphocyte–monocyte ratio, PLR platelet–lymphocyte ratio, LDH lactate dehydrogenase, β2m β 2-microglobulin, OS overall survival, PFS progression-free survival, HR hazard ratio
Discussion
Primary gastrointestinal diffuse large B-cell lymphoma is the most common extranodal site involved by diffuse large B-cell lymphoma [12]. The international prognostic index (IPI) developed for DLBCL is, at present, the most valuable and widely used for the stratification of almost all subtypes of NHL. Because of ignoring the host adaptive immunity and tumor microenvironment which play an important role in the pathogenesis of lymphoma, the guidance mode cannot really distinguish high-risk populations with the use of rituximab. Gene expression profiling studies in NHL, shows that gene expression by tumor-infiltrating lymphocytes and myeloid-derived cells can predict clinical outcomes [9, 13, 14]. Therefore, these findings give us evidence that peripheral lymphocyte counts and monocyte counts, which were biomarkers of host immunity and the tumor microenvironment, can predict the prognosis of patients. Our study showed that the correlations of ALC, AMC, LMR and PLR levels with the prognosis of PGI-DLBCL.
A total of 173 patients in this study,We found that lower ALC and LMR, higher AMC and PLR were indicators of poor prognosis.
Lymphocytes act as basic components of the immune system. Reducing lymphocytes in the blood and in the tumor stroma lead to a down regulation of the immune response against the tumor. Lymphopenia before initiation of systemic treatment was noted to be a negative of clinical outcome in solid malignancies as well as in hematological malignancies [7, 15].The important contribution of T and NK cells in immune surveillance, illustrated by occurrence of post-transplant lymphoproliferative disease and HIV-associated lymphomas. Lymphocytopenia was found to be a strong negative prognostic marker which correlated strongly with the disease burden, patients’ fitness and overall outcome. Thus, lymphopenia is considered as a sign of the host immune deficiency [16]. In our study, the univariate analysis of prognostic parameters showed that ALC was significantly associated with OS and PFS. In multivariate analysis, ALC was related with PFS, but not associated with the OS.
Monocyte, which is an important source of soluble mediators, plays an important role in suppress adaptive immunity and promotes angiogenesis, invasion, migration, and tumor growth [17, 18]. In addition, monocyte-derived cells may also provide nutrition factors that directly promote the growth and survival of malignant lymphocytes. Peripheral blood monocytes and their progeny within the tumor microenvironment express the T-cell co-inhibitory ligand B7-H1 (PD-L1), which stimulates the expansion of suppressive regulatory T cells. Therefore it is not surprising that peripheral blood monocytosis is adverse prognostic factor in various tumors [15–17]. Platelets, an important factor in thrombosis, has been known as mediate cancer cell growth, dissemination and angiogenesis. Activated platelets are able to interact with tumor cells through paracrine signaling or direct contact, thus promoting cancer cell growth and survival. Clinical data also suggested that elevated platelet number was related with poor prognosis in many types of tumors [4, 18–22]. We retrospectively analyzed all parameters in 173 patients and found that the univariate analysis of prognostic parameters showed that AMC was significantly associated with OS and PFS. However, in multivariate analysis, AMC has less significance of OS and PFS.
The LMR as a surrogate marker of host immunity (ALC) and tumor-microenvironment (AMC) has been reported as prognostic factor of clinical outcomes in DLBCL. Our study showed that LMR is also an effective prognostic factor in PGI- DLBCL patients. Besides,we found PLR’s significant role in the diagnosis of all kinds of tumors [18–22], PLR was generally high in tumor patients and patients with higher value of PLR had lower survival. Our findings suggested that patients with an PLR > 170 in PGI-DLBCL might be lower OS and PFS.
The gene expression profiling (GEP) of lymphoma cells is another Important factor that influences the prognosis of PGI-DLBCL patients. According to GEP,three gene-expression subgroups-germinal-center B-cell–like (GCB), activated B-cell–like (ABC), and type 3 diffuse large-B-cell lymphoma—were demonstrated [23]. Although a quite powerful tool that reveals wide-ranging biological information about tumor biology, gene expression profiling is not easily available in most laboratories. Immunohistochemistry stain is readily available and routinely performed. It is used to divided DLBCL into GCB and non-GCB groups [24]. Studies have reported that GCB phenotype has a significantly better prognosis compared to patients with the non-GCB phenotype [25].In our study, both PFS and OS data were not significantly different between the two groups.
Conclusion
In summary, although the means of evaluating PGI-DLBCL prognosis are varied, but these tools have some flaws. IPI totally overlooked the host immunity and tumor microenvironment which are important to the pathogenesis of lymphoma. The price of immunohistochemical stain is very high and sampling method is difficultly. Positron Emission Tomography/Computed Tomography (PET/CT) is not only expensive but also increases the number of false positive. The Peripheral blood cell count, readily available, inexpensive, objective data, is expected to become a means to evaluate the prognosis of PGI-DLBCL patients.
Acknowledgments
I would like to express my gratitude to all those who helped me during the writing of this thesis. I gratefully acknowledgement the help of my supervisor, Dr. Xiaofang Wang, for her constant encouragement and guidance. Also, I owe much to Miss Li Zang for her valuable suggestion and critiques which are of help and important in making the thesis a reality. This research was supported by grants from the National Natural Science Foundation of China (No. 81272562).
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number 81272562).
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Delfa Radic-Krito APP, Ostojic S, Vrhovac R, Kardum-Skelin I, Jakcs B. Primary gastrointestinal non-Hodgkin lymphoma in adults: clinicopathologic and survival characteristics. Coll Antropol. 2010;34(2):413–417. [PubMed] [Google Scholar]
- 2.Erkurt MA, Aydogdu I, Kuku I, Kaya I, Basaran Y. Clinicopathologic characteristics and therapeutic outcomes of primary gastrointestinal non-Hodgkin’s lymphomas: 10 years of experience from a single center in eastern Anatolia. Med Princ Pract. 2009;18(5):399–406. doi: 10.1159/000226295. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Wang W, Wang J, Jing H, Jijun W, Liu Y, Zhao W, Ke X. Clinicopathological characteristics and prognostic analysis of 92 cases with primary gastrointestinal diffuse large B-cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2014;35(4):288–294. doi: 10.3760/cma.j.issn.0253-2727.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Gunaldi M, Goksu S, Erdem D, Gunduz S, Ozdem Y, Inan YO, Tiken E, Kahraman S, Inan YO, Genc TB, Yildirim M. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. 2015;8(4):5937–5942. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Wang L, Yu D, Shen Y, Cheng S, Zhang L, Qian Y, Shen Z, Li Q, Zhao W. Localized primary gastrointestinal diffuse large B cell lymphoma received a surgical approach: an analysis of prognostic factors and comparison of staging systems in 101 patients from a single institution. World J Surg Oncol. 2015;13:246. doi: 10.1186/s12957-015-0668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H, Zhang LE, Guo S, Yuan T, Xia B, Zhang L, Zhang Y. Overexpression of DNA methyltransferase 1 as a negative independent prognostic factor in primary gastrointestinal diffuse large B-cell lymphoma treated with CHOP-like regimen and rituximab. Oncol Lett. 2015;9(5):2307–2312. doi: 10.3892/ol.2015.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YL, Gu KS, Pan YY, Jiao Y, Zhai ZM. Peripheral blood lymphocyte/monocyte ratio at the time of first relapse predicts outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. BMC Cancer. 2014;14(1):1. doi: 10.1186/1471-2407-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lia YL, Gu KS, Panb YY, Jiaob Y, Zhaia ZM. The lower peripheral blood lymphocyte/monocyte ratio assessed during routine follow-up after standard first-line chemotherapy is a risk factor for predicting relapse in patients with diffuse large B-cell lymphoma. Leuk Res. 2014;38(3):323–328. doi: 10.1016/j.leukres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte–monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(10):e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin B, Chen C, Qian Y, Feng J. Prognostic role of peripheral blood lymphocyte/monocyte ratio at diagnosis in diffuse large B-cell lymphoma: a meta-analysis. Leuk Lymphoma. 2015;9(56):1–6. doi: 10.3109/10428194.2015.1014367. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1–IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8:1355–1362. doi: 10.2147/OTT.S82174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Gui W, Shen Q. Primary gastrointestinal non-Hodgkin’s lymphoma: clinicopathological and prognostic analysis. Med Oncol. 2010;27(3):661–666. doi: 10.1007/s12032-009-9265-1. [DOI] [PubMed] [Google Scholar]
- 13.Azambuja D, Natkunam Y, Biasoli I. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin’s lymphoma.pdf. Ann Oncol. 2011;23(3):736–742. doi: 10.1093/annonc/mdr157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS, Thompson C, Markovic SN. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97(2):262–269. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Feng JF. Low preoperative lymphocyte–monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8:137–145. doi: 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C, Go H, Kim JE, Kim CW, Huh J. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17:871–880. doi: 10.1634/theoncologist.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibutani M, Maeda K, Nagahara H. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21(34):9966–9973. doi: 10.3748/wjg.v21.i34.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye X, Chen Y, Chen D, Dettke M. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumor Biol. 2015;36:9319–9325. doi: 10.1007/s13277-015-3667-9. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Hu H, Gu S. Platelet to lymphocyte ratio plays an important role in prostate cancer’s diagnosis and prognosis. Int J Clin Exp Med. 2015;8(7):11746–11751. [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Han Z, Cheng Z, Yu J, Yu X, Liang P. Clinical significance of preoperative platelet-to-lymphocyte ratio in recurrent hepatocellular carcinoma after thermal ablation: a retrospective analysis. Int J Hyperth. 2015;31:758–763. doi: 10.3109/02656736.2015.1068958. [DOI] [PubMed] [Google Scholar]
- 21.Sun W, Zhang L, Luo M, Hu G, Mei Q, Liu D, Long G, Hu G. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet–lymphocyte ratio. Head Neck. 2015;38(51):E1332–E1340. doi: 10.1002/hed.24224. [DOI] [PubMed] [Google Scholar]
- 22.Shirai Y, Shiba H, Sakamoto T, Horiuchi T, Haruki K, Fujiwara Y, Futagawa Y, Ohashi T, Yanaga K. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158(2):360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 24.Haarer CF, Roberts RA, Frutiger YM, Grogan TM, Rimsza LM. Immunohistochemical classification of de novo, transformed, and relapsed diffuse large B-cell lymphoma into germinal center B-cell and nongerminal center B-cell subtypes correlates with gene expression profile and patient survival. Arch Pathol Lab Med. 2006;130(12):1819–1824. doi: 10.5858/2006-130-1819-ICODNT. [DOI] [PubMed] [Google Scholar]
- 25.Nyman HA, Karjalainen-Lindsberg M, Taskinen ML, Berglund M, Amini RM, Blomqvist C, Enblad G, Leppa S. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109(11):4930–4935. doi: 10.1182/blood-2006-09-047068. [DOI] [PubMed] [Google Scholar]